The Duke Human Heart Repository began in earnest in 2008 as an investigator driven entity designed to address key questions of interest in the Milano/Bowles research program related to cardiovascular disease causes and treatments, and improvement of cardiac transplantation and mechanical circulatory support outcomes.
Our self-imposed mission statement is as follows:
- To collect and store cardiac and related tissues (blood derivatives plasma/serum), urine, and clinical information on subjects undergoing ventricular assist device implantation, heart transplantation, or other cardiac surgeries where discarded cardiac tissue is available.
- To make these tissues available to be used in future research studies by investigators internal and external to Duke.
Our specimens are now provided through collaborations to researchers in academia and industry all over the world in projects involving cardiovascular physiology, disease, bio-markers, and molecular targets for intervention as well as in secondary pharmacological testing of pharmaceutical agents in development.
Resource Overview
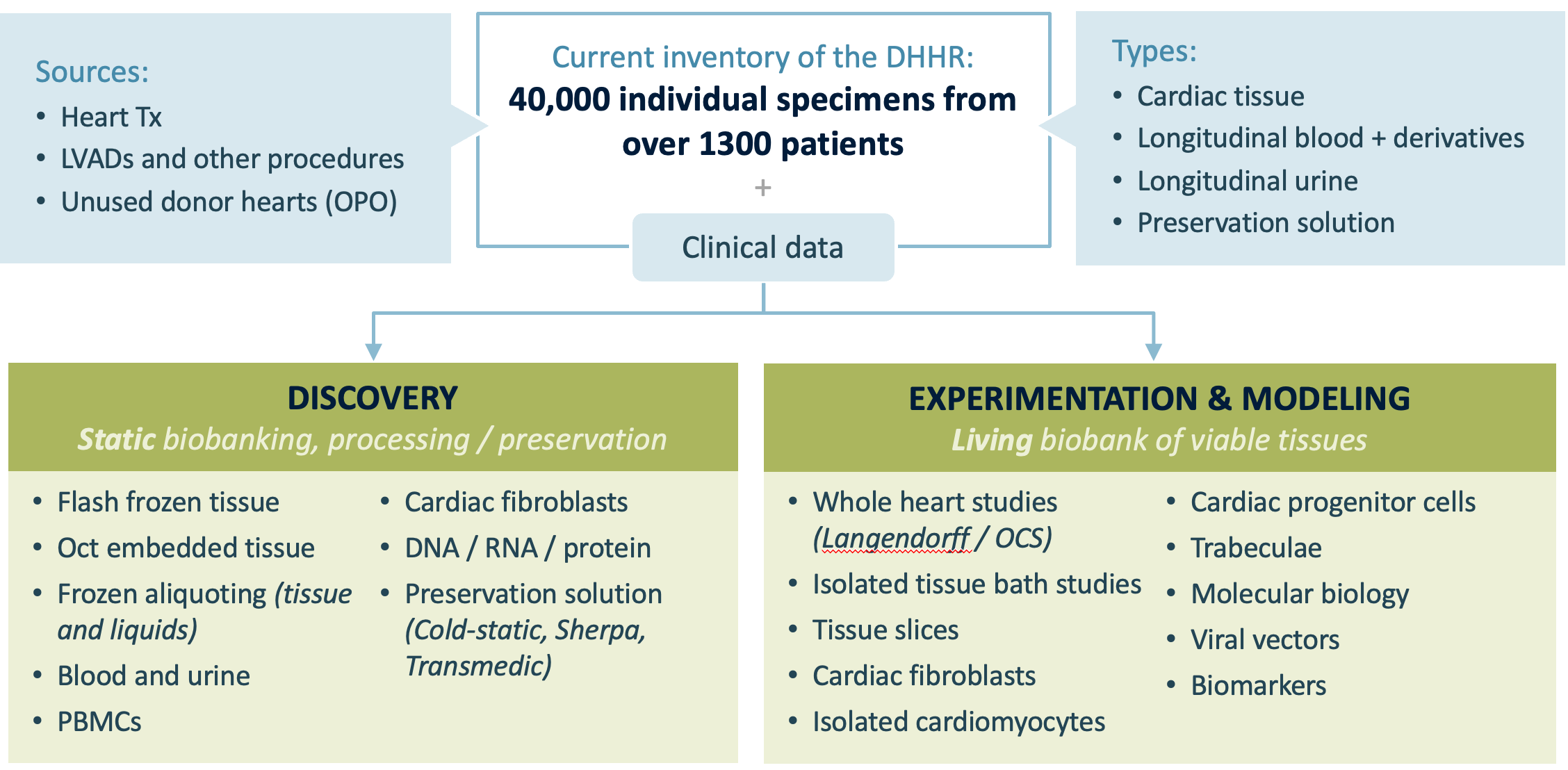
Tissues are used for discovery and models:
- Flash frozen in liquid N2 and stored at -80°C.
- Embedded in OCT histology media
- Preserved in RNAlater
- Powdered frozen tissue
- Blood and urine
- Preservation solution
Members
- Carmelo A. Milano, MD, Co-Director
- Dawn E. Bowles, PhD, Co-Director
- Jeffrey Keenan, MD
- Jacob N. Schroder, MD
- Chetan M. Patel, MD
- Christopher Holley, MD, PhD
- Ryan Gross, Repository Manager
- Chunbo Wang, PhD
- Alejandro Lobo, MD
- Krish Dewan, MD
Selected Publications
- Crow S, Milano C, Joyce L, Chen D, Arepally G, Bowles D, Thomas W, Ortiz NV. Comparative analysis of von Willebrand factor profiles in pulsatile and continuous left ventricular assist device recipients. ASAIO J, 56(5):441-5, Sep-Oct, 2010. PubMed PMID: 20613494.
- Crow S, Chen D, Milano C, Thomas W, Joyce L, Piacentino V 3rd, Sharma R, Wu J, Arepally G, Bowles D, Rogers J, Villamizar-Ortiz N. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg, 90(4):1263-9, Oct 2010; discussion 1269. PubMed PMID: 20868825.
- Piacentino V 3rd, Milano CA, Bolanos M, Schroder J, Messina E, Cockrell AS, Jones E, Krol A, Bursac N, Mao L, Devi GR, Samulski RJ, Bowles DE. X-linked inhibitor of apoptosis protein-mediated attenuation of apoptosis, using a novel cardiac-enhanced adeno-associated viral vector. Hum Gene Ther, 23(6):635-46, Jun 2012. PubMed PMID: 22339372; PubMed Central PMCID: PMC3392616.
- Schechter MA, Hsieh MK, Njoroge LW, Thompson JW, Soderblom EJ, Feger BJ, Troupes CD, Hershberger KA, Ilkayeva OR, Nagel WL, Landinez GP, Shah KM, Burns VA, Santacruz L, Hirschey MD, Foster MW, Milano CA, Moseley MA, Piacentino V 3rd, Bowles DE. Phosphoproteomic profiling of human myocardial tissues distinguishes ischemic from non-ischemic end stage heart failure. PLoS One, 9(8):e104157, Aug 2014. PubMed PMID: 25117565; PubMed Central PMCID: PMC4130503.
- Ahmad T, Wang T, O'Brien EC, Samsky MD, Pura JA, Lokhnygina Y, Rogers JG, Hernandez AF, Craig D, Bowles DE, Milano CA, Shah SH, Januzzi JL, Felker GM, Patel CB. Effects of left ventricular assist device support on biomarkers of cardiovascular stress, fibrosis, fluid homeostasis, inflammation, and renal injury. JACC Heart Fail, 3(1):30-9, Jan 2015. PubMed PMID: 25447345.
- Pratico, ED, Feger, BJ, Boczkowski, D, Sullenger, B, Bowles, DE, Milano, C. and SK Nair. RNA-mediated reprogramming of primary adult human dermal fibroblasts into c-kit+ cardiac progenitor cells (CPCs). Stem Cells Dev, 24(22):2622-33, Nov 15, 2015. PubMed PMID: 26176491.
- Ahmad T, Kelly JP, McGarrah RW, Hellkamp AS, Fiuzat M, Testani JM, Wang TS, Verma A, Samsky MD, Donahue MP, Ilkayeva OR, Bowles DE, Patel CB, Milano CA, Rogers JG, Felker GM, O'Connor CM, Shah SH, Kraus WE. Prognostic Implications of Long-Chain Acylcarnitines in Heart Failure and Reversibility with Mechanical Circulatory Support. J Am Coll Cardiol, 67(3):291-9, Jan 26, 2016. PMID: 26796394.
- Schechter M, Watson M, Feger B, Southerland K, Mishra R, Dibernardo L, Kuchibhatla M, Schroder J, Daneshmand M, Patel C, Rogers J, Milano C, Bowles D. Elevated Cardiac Troponin I in Preservation Solution is Associated with Primary Graft Dysfunction. J Card Fail, 22(2):158-62, Feb 2016. PubMed PMID: 26365053.
- Jensen BC, Bultman, SJ, Holley D, Tang W, deRidder G, Pizzo S, Bowles D, Willis MS. Upregulation of autophagy genes and the unfolded protein response in human heart failure. Int J Clin Exp Med, 10(1):1051-1058, Jan 2017. ISSN: 1940-5901/IJCEM0040108.
- Jean-Charles P-Y, Yu SM-W, Abraham D, Kommaddi RP, Mao L, Strachan R, Zhang Z-S, Stiber J, Bowles DE, Jones S, Koch W, Rockman HA and Shenoy SK. Mdm2 regulates cardiac contractility by inhibiting GRK2-mediated desensitization of β-adrenergic receptor signaling. JCI Insight, 2(17), Sep 7, 2017. pii: 95998. doi: 10.1172/jci.insight.95998. PMID: 28878120.
- Wang T, O’Brien EC, Rogers JG, Jacoby DL, Chen ME, Testani JM, Bowles DE, Milano CA, Felker GM, Patel CB, Bonde PN, Ahmad T. Plasma levels of microRNA-155 are upregulated with long-term left ventricular assist device support. ASAIO J, 63(5):536-541, Sep/Oct 2017. PMID 28319523.
- Hayashi H, Hess DT, Zhang R, Bowles DE, Milano CA, and Stamler JS. S-nitrosylation of β-arrestins biases signaling through G protein-coupled receptors and confers ligand independence. Mol Cell, 70(3):473-487.e6, May 3, 2018. doi: 10.1016/j.molcel.2018.03.034. PMID: 29727618
- Beak JY, Kang HS, Huang W, Myers PH, Bowles DE, Jetten AM5 and Jensen B. The nuclear receptor RORα protects against angiotensin II-induced cardiac hypertrophy and heart failure. Am J Physiol Heart Circ Physiol, 316(1):H186-H200, Jan 1, 2019
- Stevenson MD, Canugovi C, Vendrov AE, Hayami T, Bowles DE, Krause K-H, Madamanchi NR and Runge MS. NADPH Oxidase 4 Regulates Inflammation in Ischemic Heart Failure: Role of Soluble Epoxide Hydrolase. Antiox Redox Sign, 31(1):39-58, Jul 2019.
- Truby LK, Kwee LC, Agarwal R, Grass E, DeVore AD, Patel CB, Chen D, Schroder JN, Bowles D, Milano CA, Shah SH, Holley CL. Proteomic profiling identifies CLEC4C expression as a novel biomarker of primary graft dysfunction after heart transplantation. J Heart Lung Transplant. 2021 Dec;40(12):1589-1598. doi: 10.1016/j.healun.2021.07.024. Epub 2021 Aug 11. PMID: 34511330; PMCID: PMC8670564.
- Bishawi M, Bowles D, Pla MM, Oakes F, Chiang Y, Schroder J, Milano C, Glass C. PD-1 and PD-L1 expression in cardiac transplantation. Cardiovasc Pathol. 2021 Sep-Oct;54:107331. doi: 10.1016/j.carpath.2021.107331. Epub 2021 Mar 16. PMID: 33737091.
- Chunhacha P, Pinkaew D, Sinthujaroen P, Bowles DE, Fujise K. Fortilin inhibits p53, halts cardiomyocyte apoptosis, and protects the heart against heart failure. Cell Death Discov. 2021 Oct 23;7(1):310. doi: 10.1038/s41420-021-00692-w. PMID: 34689154; PMCID: PMC8542040.
- Lorenzana-Carrillo MA, Gopal K, Byrne NJ, Tejay S, Saleme B, Das SK, Zhang Y, Haromy A, Eaton F, Mendiola Pla M, Bowles DE, Dyck JRB, Ussher JR, Michelakis ED, Sutendra G. TRIM35-mediated degradation of nuclear PKM2 destabilizes GATA4/6 and induces P53 in cardiomyocytes to promote heart failure. Sci Transl Med. 2022 Nov 2;14(669):eabm3565. doi: 10.1126/scitranslmed.abm3565. Epub 2022 Nov 2. PMID: 36322626.
- Liu J, Ma P, Lai L, Villanueva A, Koenig A, Bean GR, Bowles DE, Glass C, Watson M, Lavine KJ, Lin CY. Transcriptional and Immune Landscape of Cardiac Sarcoidosis. Circ Res. 2022 Sep 16:101161CIRCRESAHA121320449. doi: 10.1161/CIRCRESAHA.121.320449. Epub ahead of print. PMID: 36111531.
- Lozhkin A, Vendrov AE, Ramos-Mondragón R, Canugovi C, Stevenson MD, Herron TJ, Hummel SL, Figueroa AC, Bowles DE, Isom LL, Runge MS, Madamanchi NR, Mitochondrial oxidative stress contributes to diastolic dysfunction through impaired mitochondrial dynamics, Redox Biol. 2022 Nov;57:102474. doi:10.1016/j.redox.2022.102474. Epub 2022 Sep 17. PMID: 36183542; PMCID: PMC9530618.
- Fassler M, Tshori S, Barac Y, Bowles DE, Benaim C, George J. Dual Targeting of Soluble Oligomeric and Aggregated Transthyretin with a Monoclonal Antibody Ameliorates Experimental Neuropathy. Biology (Basel). 2022 Oct 15;11(10):1509. doi: 10.3390/biology11101509. PMID: 36290413; PMCID: PMC9598441.
- Chakraborty A, Peterson NG, King JS, Gross RT, Mendiola Pla M, Thennavan A, Zhou KC, DeLuca S, Bursac N, Bowles DE, Wolf MJ, Fox DT Conserved Chamber-Specific Polyploidy Maintains Heart Function in Drosophila. bioRxiv [Preprint]. 2023 Feb 11:2023.02.10.528086. doi: 10.1101/2023.02.10.528086. Update in: Development. 2023 Aug 01;: PMID: 36798187; PMCID: PMC9934670.
- Mohammed M, Ogunlade B, Elgazzaz M, Berdasco C, Lakkappa N, Ghita I, Guidry JJ, Sriramula S, Xu J, Restivo L, Mendiola Plá MA, Bowles DE, Beyer AM, Yue X, Lazartigues E, Filipeanu CM. Nedd4-2 up-regulation is associated with ACE2 ubiquitination in hypertension. Cardiovasc Res. 2023 Sep 5;119(11):2130-2141. doi: 10.1093/cvr/cvad070. PMID: 37161607; PMCID: PMC10478751.
- Truby LK, Kwee LC, Bowles DE, Casalinova S, Ilkayeva O, Muehlbauer MJ, Huebner JL, Holley CL, DeVore AD, Patel CB, Kang L, Pla MM, Gross R, McGarrah RW, Schroder JN, Milano CA, Shah SH. Metabolomic profiling during ex situ normothermic perfusion before heart transplantation defines patterns of substrate utilization and correlates with markers of allograft injury. J Heart Lung Transplant. 2024 May;43(5):716-726. doi: 10.1016/j.healun.2023.12.002. Epub 2023 Dec 7. PMID: 38065238.
- Zhu Q, Combs ME, Bowles DE, Gross RT, Mendiola Pla M, Mack CP, Taylor JM. GRAF1 Acts as a Downstream Mediator of Parkin to Regulate Mitophagy in Cardiomyocytes. Cells. 2024 Mar 4;13(5):448. doi: 10.3390/cells13050448. PMID: 38474413; PMCID: PMC10930636.
- Zhu Q, Combs ME, Liu J, Bai X, Wang WB, Herring LE, Liu J, Locasale JW, Bowles DE, Gross RT, Pla MM, Mack CP, Taylor JM. GRAF1 integrates PINK1-Parkin signaling and actin dynamics to mediate cardiac mitochondrial homeostasis. Nat Commun. 2023 Dec 11;14(1):8187. doi: 10.1038/s41467-023-43889-6. PMID: 38081847; PMCID: PMC10713658.
- Karpurapu A, Williams H, DeBenedittis P, Baker CE, Ren S, Thomas MC, Beard AJ, Devlin GW, Harrington J, Parker LE, Smith AK, Mainsah M, Pla MM, Asokan A, Bowles DE, Iversen E, Collins L, Karra R. Deep Learning Resolves Myovascular Dynamics in the Failing Human Heart. J Am Coll Cardiol Basic Trans Science. 2024 May, 9 (5) 674–686. https://doi.org/10.1016/j.jacbts.2024.02.007.
- Lorenzana-Carrillo MA *, Tejay S *, Nanoa J, Huang G, Liu Y, Haromy A, Zhao YY, Mendiola Pla M, Bowles DE, Kinnaird A, Michelakis ED, Sutendra G. TRIM35 Monoubiquitinates H2B in Cardiac Cells, Implications for Heart Failure Circulation Research. 2024;135:00–00. DOI: 10.1161/CIRCRESAHA.123.324202
- Elgazzaz M, Lakkappa N, Berdasco C, Mohan UP, Nuzzo A, Restivo L, Martinez A, Scarborough A, Guidry JJ, Sriramula S, Xu J, Daoud H, Mendiola Plá MA, Bowles DE, Beyer AM, Mauvais-Jarvis F, Yue X, Filipeanu CM, Lazartigues E. UBR1 Promotes Sex-Dependent ACE2 Ubiquitination in Hypertension. medRxiv [Preprint]. 2024 May 25:2024.05.23.24307722. doi: 10.1101/2024.05.23.24307722. PMID: 38826318; PMCID: PMC11142264.
Collaborate with Us
For additional information or for sample requests please contact:
Dawn Bowles, PhD
Assistant Professor in Surgery
dawn.bowles@duke.edu