Overview

The Duke Ex Vivo Organ Laboratory (DEVOL) is an integrative research laboratory exploring the transformative potential of ex vivo machine perfusion (EVMP), which keeps organs viable by pumping blood through them rather than keeping them on ice. The lab is dedicated to exploring two facets of EVMP: how it can enhance the quality of abdominal and thoracic organs for transplant, and how it can be an investigative platform for the selective study of organ systems.
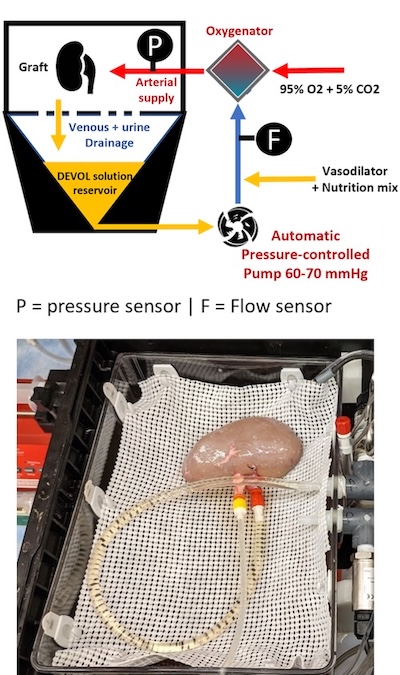
Transplantation remains the gold standard treatment for most forms of end-stage organ failure, but with more than 180,000 patients in the U.S. awaiting a heart, intestine, liver, lung, or kidney transplant in a given year, the demand for organs far exceeds the available supply of suitable grafts. Improving how well, and how long, donor organs are preserved can enhance the function and viability of organs that would otherwise need to be discarded. It can also substantially increase patient access to transplantation. As a result, EVMP platforms aim to tailor perfusion conditions to meet the particular metabolic demands of the graft, extending preservation and promoting rehabilitation of the graft. This provides an ideal environment for the development and/or administration of precision medicines to treat otherwise intractable disease. This precise degree of control over operating conditions makes EVMP an ideal investigative tool for studying the fundamentals of organ function with unparalleled specificity.
Recognizing EVMP’s multifaceted value proposition, the DEVOL uses cutting-edge approaches in the fields of biochemistry, bioengineering, immunology, and virology to evolve EVMP design. This includes engineering new solutions for perpetual graft preservation and applying next-generation therapeutics, including viral vector-mediated gene therapies to enhance graft longevity after transplant.
To conduct these studies, the laboratory uses small and large animal transplantation models that offer an exceptional training environment for surgical residents and biomedical scientists. Those interested in participating are encouraged to contact Andrew Barbas, MD, Matthew Hartwig, MD, or Ben Hughes, PhD, directly about research opportunities.
Research Highlights
Perpetual EVMP to Enhance Graft Health
Creative engineering and biochemical solutions are key to long-term graft preservation.
EVMP-Administered Next-Generation Therapeutics
Viral vector-mediated gene therapies are a paradigm shift in the treatment of intractable diseases, and EVMP is an ideal delivery platform.
Interrogating Organ Function During EVMP
Organs are made up of a rich diversity of cell types and, in the case of lungs and intestines, active microbial ecosystems. This makes EVMP an exceptional way to study the functional relationships among those cells without the background biological processes that can often confound in vivo investigations.
Research
Areas of focus include:
Perpetual EVMP to Enhance Graft Health
Creative engineering and biochemical solutions are key to long-term graft preservation.
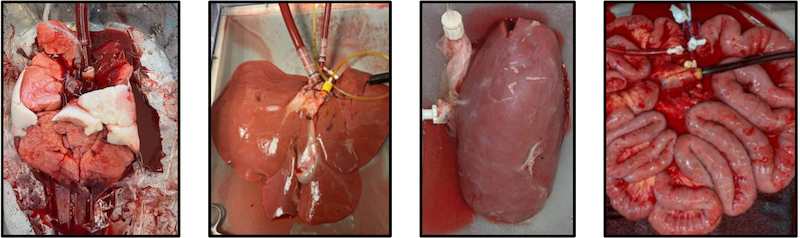
Ex vivo machine perfusion (EVMP) platforms can operate at different temperature ranges, depending on the user’s desire to promote or inhibit cellular metabolism. Normothermic temperatures run at around 37ºC, and hypothermic temperatures are in the 4-10ºC range. As an alternative, sub-normothermic (~20-30ºC) temperatures offer key advantages, including eliminating heater/cooler units, which reduces the complexity of the circuit. Perhaps most importantly, the lower metabolic demand with sub-normothermic temperatures means that there is no need for perfusate supplementation with an active oxygen carrier, typically in the form of added red blood cells. This relieves competition for a frequently scarce resource at health centers, and avoids the potential interference of therapeutic biologics like adeno-associated viruses with the red blood cells. Our laboratory has conducted successful tests of SNMP in rodent liver, lung, and kidney perfusions, and more recently demonstrated the superiority of SNMP over static cold storage using a porcine model of kidney auto-transplantation.
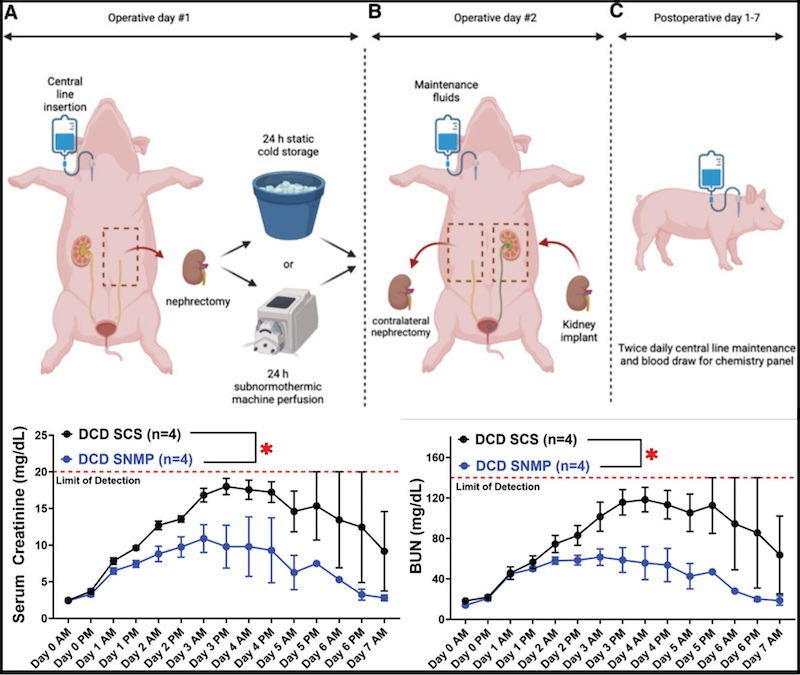
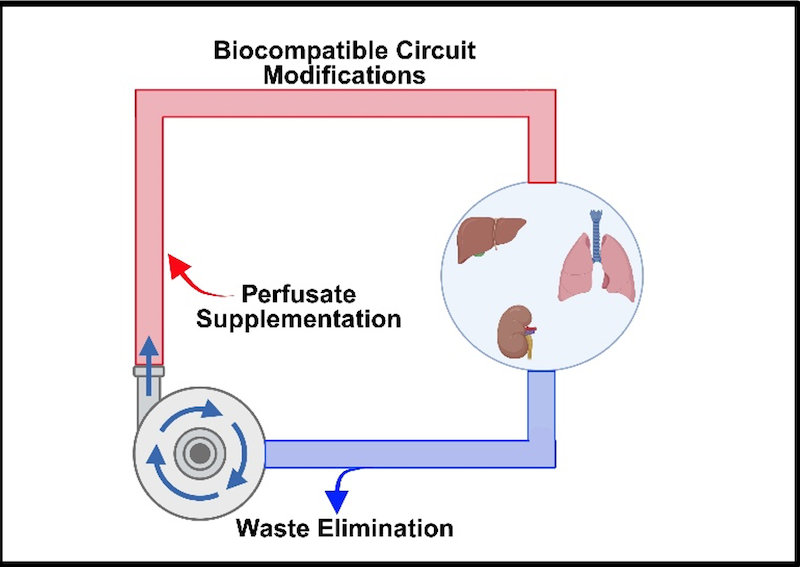
A core goal of the laboratory is demonstrating multi-day, if not indefinite, organ preservation by machine perfusion. There is considerable value in achieving perpetual organ preservation, including simplified transplant procedure scheduling and sufficient time for extensive donor and recipient matching. Most importantly, though, extending the duration of perfusion allows for “personalized medicine” to treat graft injuries, improving viability and reducing post-operative complications. Maintaining perfusate viability over this interval is key to long-term perfusion, requiring periodic re-supplementation with metabolic substrates as well as efficient waste elimination. However, direct fluid removal and replenishment is expensive and can increase the likelihood of adverse events such as contamination during perfusion.
Our laboratory is actively engaged in engineering solutions for these and other factors that can be obstacles to long-term perfusion. Among these are modified integrated dialysis circuits, bespoke perfusates for the management of tissue edema, and alternative circuit components including low-hemolysis pumps. For particularly sensitive organs like the lungs, manifestation of pressure necrosis resulting from static organ positioning is a key concern, and ongoing experiments are investigating novel solutions to relieve this and dependent edema. Using modified protocols, we have thus far achieved successful liver, kidney, and intestine transplants following up to 24 hours of machine perfusion. By incorporating more recent evolutions in device design and methodology, multi-day continuous perfusion appears imminent.
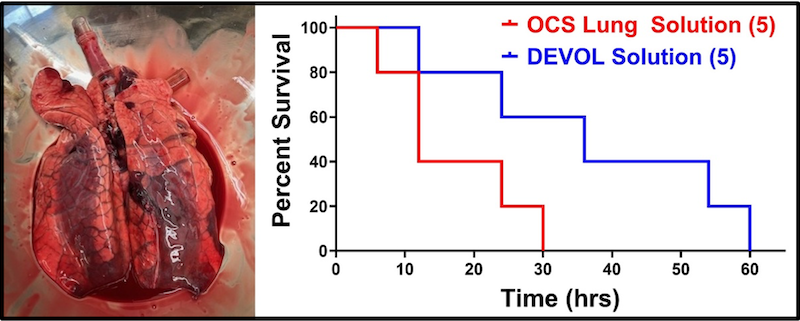
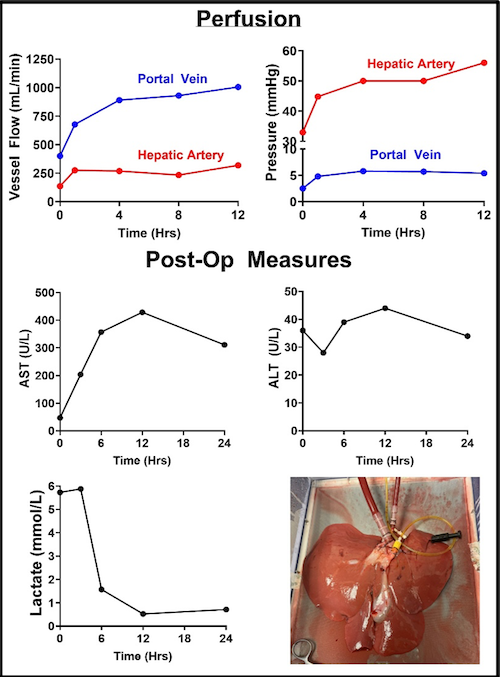
EVMP-Administered Next-Generation Therapeutics
Viral vector-mediated gene therapies are a paradigm shift in the treatment of intractable diseases, and EVMP is an ideal delivery platform.
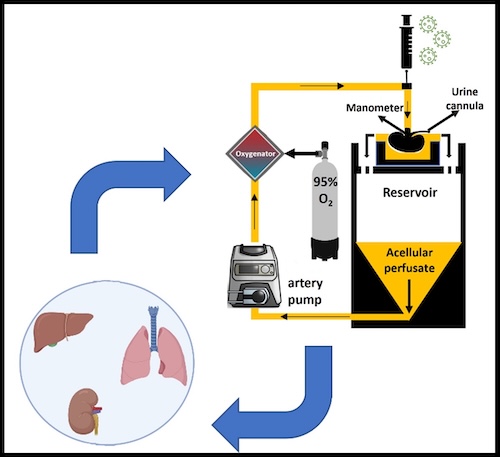
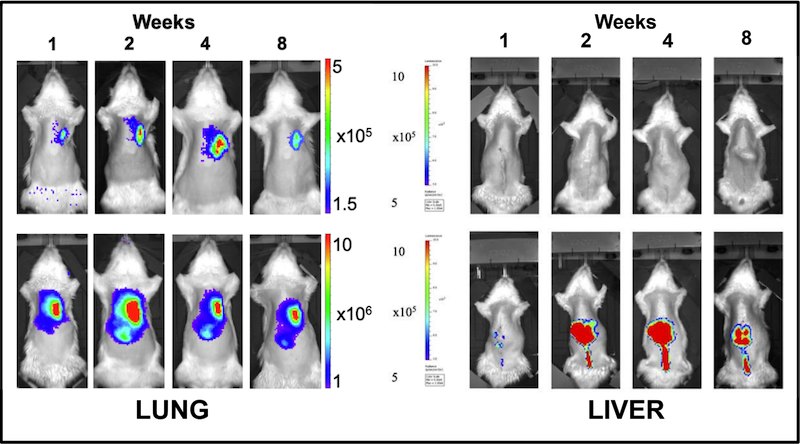
The advent of adeno-associated virus (AAV) vectors has led to a renaissance in the development and use of gene therapeutics, due in large part to the exceptional safety of AAV. Ex vivo machine perfusion is an ideal method for the delivery of gene therapeutics, including AAV, owing to the isolated nature of the graft that effectively eliminates concerns of off-target gene expression (Figure X). Our laboratory leverages both, with the goal of engineering donor grafts with exceptional resiliency. We have performed a number of concurrent studies examining gene augmentation for the amelioration of post-transplant rejection.
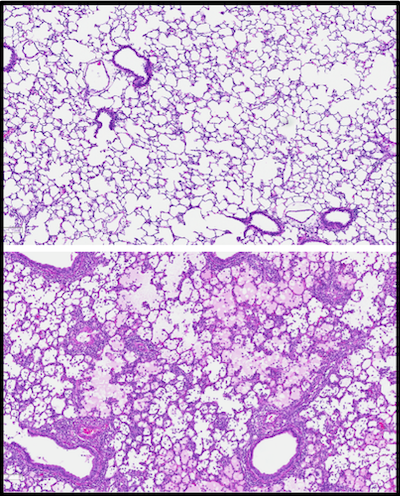
Current strategies to reduce organ rejection involve the use of immunosuppressive agents, which can incur significant side effects. Using AAV vectors, we are exploring the potential of anti-inflammatory protein over-expression to mask grafts from the recipient immune system. As shown in Figure Y, acute cellular rejection is characterized by infiltration of recipient T cells that attack donor tissue, promoting graft failure. Preclinical studies have shown promise, and further modifications to both the method of administration (such as the route of virus instillation and perfusion operating parameters) are expected to enhance transgene expression with commensurate increases in therapeutic efficacy. An additional line of investigation seeks to direct expression into particular cell types, leveraging cell subtype-specific capsids, gene promotors, and routes of administration to enrich expression in the cellular compartment of interest (Figure Z, A).
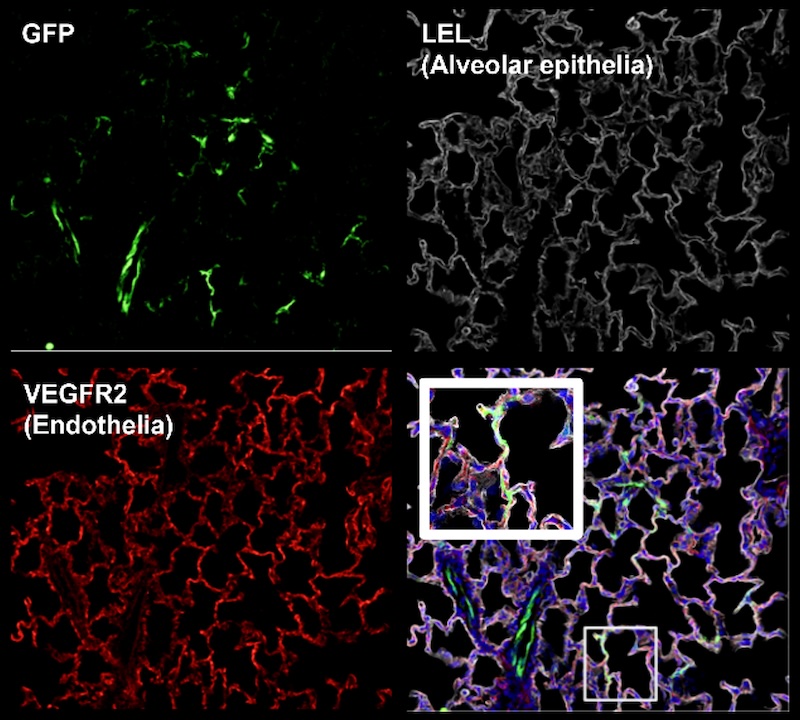
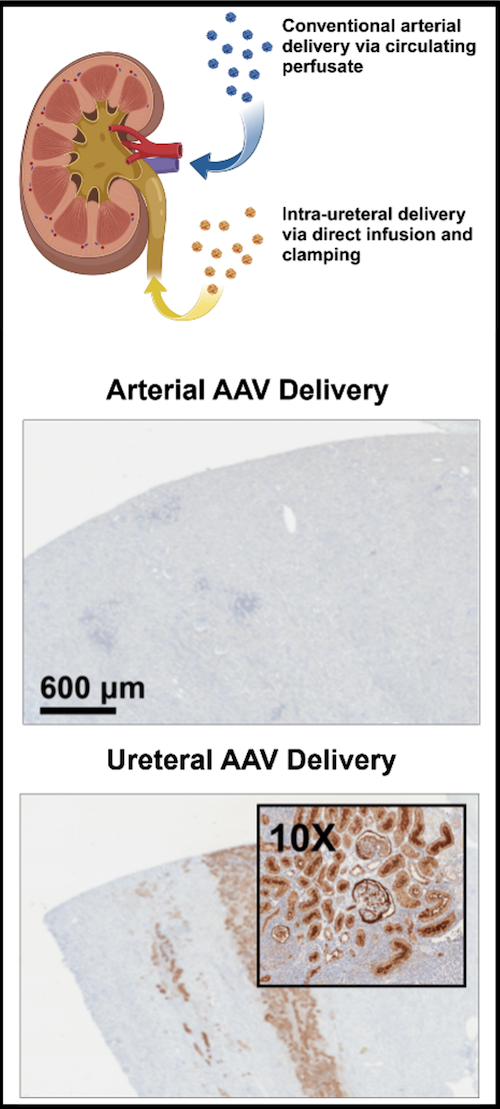
Interrogating Organ Function During EVMP
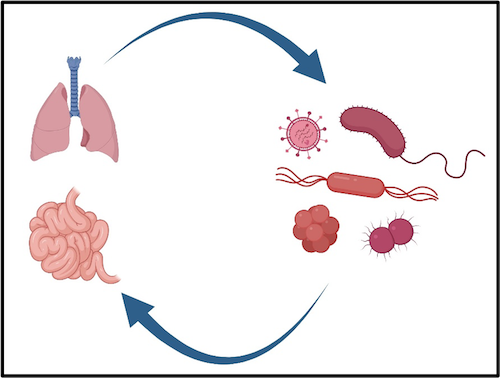
Organs are made up of a rich diversity of cell types and, in the case of lungs and intestines, active microbial ecosystems. This makes EVMP an exceptional way to study the functional relationships among those cells without the background biological processes that can often confound in vivo investigations.
The reductive environment of EVMP gives exceptional control over preservation and facilitates tailored treatment regimens for recuperating damaged organs. In addition, this approach offers the opportunity to examine intact organ function with unparalleled specificity. Our laboratory is conducting a range of studies that leverage EVMP as an investigational platform, including tests of novel compounds for the abrogation of ischemia-reperfusion injury (IRI), identification of robust metabolic indicators of graft function and health, and ongoing experiments delineating the expansion and migration of passenger immune cells during and after EVMP. Observations from these studies could be instrumental in promoting graft recovery, as well as developing novel diagnostic tools for investigators and clinicians alike.
In addition to these questions, our interests have more recently included the contribution of resident, and recipient, microbiomes on graft function and post-operative outcomes. Both the intestine and lung host diverse populations of microbes and mycobiota that can profoundly impact patient health. EVMP is an ideal platform for investigating how changes in these biomes may directly influence the function and health of these organs with exquisite experimenter control. We are furthermore investigating how modifying the recipient biome(s) may influence the manifestation of post-transplant graft dysfunction.
People
Laboratory Affiliates
Affiliated Faculty
Selected Publications
Kahan R, Alderete IS, Gao Q, Hughes B, Zhang M, Gonzalez T, Rosales A, Carney J, Aykun N, Abraham N, Hassan A, Nie X, Song M, Nakata K, Asokan A, Barbas AS, Hartwig MG. Adeno-associated virus-mediated transduction of PD-L1 in a rodent lung transplant model. Am J Transplant. 2025 May 29:S1600-6135(25)00286-2. doi: 10.1016/j.ajt.2025.05.029. Epub ahead of print. PMID: 40449603.
Nakata K, Alderete IS, Hughes BA, Hartwig MG. Ex vivo lung perfusion: recent advancements and future directions. Front Immunol. 2025 Feb 24;16:1513546. doi: 10.3389/fimmu.2025.1513546. PMID: 40066445; PMCID: PMC11891253.
Rosales A, Blondel LO, Hull J, Gao Q, Aykun N, Peek JL, Vargas A, Fergione S, Song M, Wilson MH, Barbas AS, Asokan A. Evolving adeno-associated viruses for gene transfer to the kidney via cross-species cycling of capsid libraries. Nat Biomed Eng. 2025 Feb 5. doi: 10.1038/s41551-024-01341-0. Epub ahead of print. PMID: 39910375.
Gouchoe DA, Sanchez PG, D'Cunha J, Bermudez CA, Daneshmand MA, Davis RD, Hartwig MG, Wozniak TC, Kon ZN, Griffith BP, Lynch WR, Machuca TN, Weyant MJ, Jessen ME, Mulligan MS, D'Ovidio F, Camp PC, Cantu E, Whitson BA; NOVEL and NOVEL Extension Trial Investigators. Ex vivo lung perfusion in donation after circulatory death: A post hoc analysis of the Normothermic Ex Vivo Lung Perfusion as an Assessment of Extended/Marginal Donors Lungs trial. J Thorac Cardiovasc Surg. 2024 Sep;168(3):724-734.e7. doi: 10.1016/j.jtcvs.2024.03.011. Epub 2024 Mar 19. PMID: 38508486.
Kahan R, Abraham N, Zhang M, Novokhatny V, Alderete I, Cray P, Chen F, Gao Q, Cywinska G, Neill R, Nakata K, Hassan A, Rush C, Penaflor J, Pollara JJ, Hartwig MG, Hughes B, Barbas AS. Optimizing DCD Liver Grafts With Prolonged Warm Ischemic Time Using Stabilized Plasmin in a Static Cold Storage Orthotopic Rat Liver Transplant Model. Transplant Direct. 2024 Jul 5;10(8):e1665. doi: 10.1097/TXD.0000000000001665. PMID: 38988689; PMCID: PMC11230777.
Abraham N, Gao Q, Kahan R, Alderete IS, Wang B, Howell DN, Anwar IJ, Ladowski JM, Nakata K, Jarrett E, Hlewicki K, Cywinska G, Neill R, Aardema C, Gerber DA, Roy-Chaudhury P, Hughes BA, Hartwig MG, Barbas AS. Subnormothermic Oxygenated Machine Perfusion (24 h) in DCD Kidney Transplantation. Transplant Direct. 2024 May 24;10(6):e1633. doi: 10.1097/TXD.0000000000001633. PMID: 38807861; PMCID: PMC11132391.
Gao Q, Kahan R, Gonzalez TJ, Zhang M, Alderete IS, DeLaura I, Kesseli SJ, Song M, Asokan A, Barbas AS, Hartwig MG. Gene delivery followed by ex vivo lung perfusion using an adeno-associated viral vector in a rodent lung transplant model. J Thorac Cardiovasc Surg. 2024 May;167(5):e131-e139. doi: 10.1016/j.jtcvs.2023.08.047. Epub 2023 Sep 9. PMID: 37678606.
Ludwig EK, Abraham N, Schaaf CR, McKinney CA, Freund J, Stewart AS, Veerasammy BA, Thomas M, Cardona DM, Garman K, Barbas AS, Sudan DL, Gonzalez LM. Comparison of the effects of normothermic machine perfusion and cold storage preservation on porcine intestinal allograft regenerative potential and viability. Am J Transplant. 2024 Apr;24(4):564-576. doi: 10.1016/j.ajt.2023.10.026. Epub 2023 Oct 31. PMID: 37918482; PMCID: PMC11082874.
Chapman WC, Barbas AS, D'Alessandro AM, Vianna R, Kubal CA, Abt P, Sonnenday C, Barth R, Alvarez-Casas J, Yersiz H, Eckhoff D, Cannon R, Genyk Y, Sher L, Singer A, Feng S, Roll G, Cohen A, Doyle MB, Sudan DL, Al-Adra D, Khan A, Subramanian V, Abraham N, Olthoff K, Tekin A, Berg L, Coussios C, Morris C, Randle L, Friend P, Knechtle SJ. Normothermic Machine Perfusion of Donor Livers for Transplantation in the United States: A Randomized Controlled Trial. Ann Surg. 2023 Nov 1;278(5):e912-e921. doi: 10.1097/SLA.0000000000005934. Epub 2023 Jun 26. PMID: 37389552.
Kahan R, Cray PL, Abraham N, Gao Q, Hartwig MG, Pollara JJ, Barbas AS. Sterile inflammation in liver transplantation. Front Med (Lausanne). 2023 Aug 10;10:1223224. doi: 10.3389/fmed.2023.1223224. PMID: 37636574; PMCID: PMC10449546.
Kesseli SJ, Krischak MK, Gao Q, Gonzalez T, Zhang M, Halpern SE, Kahan R, Song M, Huffman N, Xu H, Abraham N, Asokan A, Barbas AS, Hartwig MG. Adeno-associated virus mediates gene transduction after static cold storage treatment in rodent lung transplantation. J Thorac Cardiovasc Surg. 2023 Jul;166(1):e38-e49. doi: 10.1016/j.jtcvs.2022.08.050. Epub 2022 Sep 24. PMID: 38501313.
Gao Q, Kesseli SJ, Gonzalez T, Zhang M, Kahan R, Krischak M, Halpern SE, Song M, Xu H, Abraham N, Anwar IJ, Alderete I, Asokan A, Hartwig MG, Barbas AS. AAV9-mediated gene delivery to liver grafts during static cold storage in a rat liver transplant model. Front Transplant. 2023 May 30;2:1171272. doi: 10.3389/frtra.2023.1171272. PMID: 38993865; PMCID: PMC11235296.
Hartwig MG, Klapper JA, Poola N, Banga A, Sanchez PG, Murala JS, Potenziano JL. A Randomized, Multicenter, Blinded Pilot Study Assessing the Effects of Gaseous Nitric Oxide in an Ex Vivo System of Human Lungs. Pulm Ther. 2023 Mar;9(1):151-163. doi: 10.1007/s41030-022-00209-5. Epub 2022 Dec 13. PMID: 36510099; PMCID: PMC9744669.
Delaura IF, Gao Q, Anwar IJ, Abraham N, Kahan R, Hartwig MG, Barbas AS. Complement-targeting therapeutics for ischemia-reperfusion injury in transplantation and the potential for ex vivo delivery. Front Immunol. 2022 Oct 19;13:1000172. doi: 10.3389/fimmu.2022.1000172. PMID: 36341433; PMCID: PMC9626853.
Abraham N, Ludwig EK, Schaaf CR, Veerasammy B, Stewart AS, McKinney C, Freund J, Brassil J, Samy KP, Gao Q, Kahan R, Niedzwiecki D, Cardona DM, Garman KS, Barbas AS, Sudan DL, Gonzalez LM. Orthotopic Transplantation of the Full-length Porcine Intestine After Normothermic Machine Perfusion. Transplant Direct. 2022 Oct 24;8(11):e1390. doi: 10.1097/TXD.0000000000001390. PMID: 36299444; PMCID: PMC9592306.
Gao Q, DeLaura IF, Anwar IJ, Kesseli SJ, Kahan R, Abraham N, Asokan A, Barbas AS, Hartwig MG. Gene Therapy: Will the Promise of Optimizing Lung Allografts Become Reality? Front Immunol. 2022 Jul 1;13:931524. doi: 10.3389/fimmu.2022.931524. PMID: 35844566; PMCID: PMC9283701.
Gloria JN, Yerxa J, Kesseli SJ, Davis RP, Samoylova ML, Barbas AS, Hartwig MG; Duke Ex Vivo Organ Laboratory. Subnormothermic ex vivo lung perfusion attenuates graft inflammation in a rat transplant model. J Thorac Cardiovasc Surg. 2022 Aug;164(2):e59-e70. doi: 10.1016/j.jtcvs.2021.01.066. Epub 2021 Jan 30. PMID: 33640121.
Jawitz OK, Raman V, Becerra D, Doberne J, Choi AY, Halpern SE, Klapper JA, Hartwig MG. Lung Transplantation After Ex Vivo Lung Perfusion Early Outcomes From a US National Registry. Ann Surg. 2022 May 1;275(5):1006-1012. doi: 10.1097/SLA.0000000000004233. Epub 2020 Jul 24. PMID: 32740244; PMCID: PMC9550264.
Halpern SE, Kesseli SJ, Au S, Krischak MK, Olaso DG, Smith H, Tipton G, Jamieson IR, Barbas AS, Haney JC, Klapper JA, Hartwig MG. Lung transplantation after ex vivo lung perfusion versus static cold storage: An institutional cost analysis. Am J Transplant. 2022 Feb;22(2):552-564. doi: 10.1111/ajt.16794. Epub 2021 Sep 2. PMID: 34379885; PMCID: PMC8813879.
Abraham N, Zhang M, Cray P, Gao Q, Samy KP, Neill R, Cywinska G, Migaly J, Kahan R, Pontula A, Halpern SE, Rush C, Penaflor J, Kesseli SJ, Krischak M, Song M, Hartwig MG, Pollara JJ, Barbas AS. Two Compartment Evaluation of Liver Grafts During Acellular Room Temperature Machine Perfusion (acRTMP) in a Rat Liver Transplant Model. Front Med (Lausanne). 2022 Feb 24;9:804834. doi: 10.3389/fmed.2022.804834. PMID: 35280912; PMCID: PMC8907827.
Kesseli SJ, Gloria JN, Abraham N, Halpern SE, Cywinska GN, Zhang M, Moris D, Schmitz R, Shaw BI, Fitch ZW, Song M, Guy CD, Hartwig MG, Knechtle S, Barbas AS. Point-of-Care Assessment of DCD Livers During Normothermic Machine Perfusion in a Nonhuman Primate Model. Hepatol Commun. 2021 Sep;5(9):1527-1542. doi: 10.1002/hep4.1734. Epub 2021 May 4. PMID: 34510831; PMCID: PMC8435285.
Halpern SE, Rush CK, Edwards RW, Brennan TV, Barbas AS, Pollara J. Systemic Complement Activation in Donation After Brain Death Versus Donation After Circulatory Death Organ Donors. Exp Clin Transplant. 2021 Jul;19(7):635-644. doi: 10.6002/ect.2020.0425. Epub 2021 Apr 16. PMID: 33877036.
Contact Us
The DEVOL is committed to advancing bench discoveries into clinical practice, and is always open to developing both academic and industry collaborations in service of this goal. Interested parties should contact Drs. Barbas, Hartwig, or Hughes about potential projects.
Trainees interested in joining the laboratory are encouraged to contact one of the above investigators and include their research interests and goals for fellowship.














