What Is ACCQUIREnet?
The Allied Cleft & Craniofacial Quality-Improvement and Research Network (ACCQUIREnet) is a collaborative network of multidisciplinary teams that are dedicated to standardizing outcomes assessment in cleft care. ACCQUIREnet helps teams by streamlining comprehensive data collection, aggregation, storage, and analysis. As a distributed data network, each participating site in ACCQUIREnet collects and stores its own outcomes data according to a common data model. More importantly, as a collaborative network, ACCQUIREnet fosters inter-institutional benchmarking, promotes knowledge exchange, and facilitates implementation of proven quality-improvement programs and best practices.
What Does ACCQUIREnet Do?
ACCQUIREnet facilitates standardized data collection and data capture
Participating teams agree to adhere to guidelines for prospective outcomes data collection, organized according to a standardized codebook. While teams are allowed to collect data using whatever method they prefer, almost all teams have opted to implement the REDCap®-based “CleftCap” project template developed by Duke University, which helps teams to get off the ground very quickly and ensures that data conform to a standardized, common data model.
ACCQUIREnet is a distributed data network
Each site prospectively collects data during routine clinic visits (or according to project-specific protocols). These data are stored locally (on-premises). Data queries may be distributed across the network to be run locally, or for specialized analysis data are processed through an aggregation pipeline.
ACCQUIREnet simplifies multi-site data aggregation
Because each site is collecting data according to a standardized protocol and codebook, it is very straightforward to aggregate data from multiple sites into a unified repository.
ACCQUIREnet supports quality-improvement
The primary focus of ACCQUIREnet is to perform benchmarking for outcomes comparisons and to provide each participating team with an annual performance report and/or dashboard. Benchmarking allows each participating cleft team to compare its performance against network averages and to identify strengths and weaknesses. Moreover, the network supports teams by helping them to design robust quality-improvement projects, implement them, and monitor results over time.
ACCQUIREnet enables multi-site research
The second focus of ACCQUIREnet is to encourage and enable multi-site research. ACCQUIREnet is formally registered by ClinicalTrials.gov (NCT02702869) as an observational study. Data have been collected prospectively by each participating site according to a research protocol and aggregated into a central repository. Thus, this becomes a very robust dataset that may be used for observational studies that yield “real world” evidence. While presently there is no interventional arm in ACCQUIREnet, the network has potential to conduct embedded pragmatic clinical trials in the long-term.
ACCQUIREnet is a learning health network
One characteristic that differentiates ACCQUIREnet from other research collaboratives is that it is designed as a learning health network. The system itself is designed to improve and adapt over time, based upon results of prior data analysis and in response to the needs and desires of the participating teams. Modifications to the codebook may be proposed annually by participating. These proposed modifications will be appraised by a Stewardship Committee. Any changes would be presented to the network for a period of notice-and-comment before adoption. Once formally approved, modifications to the common data model, CleftCap codebook, or data-collection guidelines and protocols are disseminated across the network under the guidance of Duke University, which serves as the coordinating center for the network.
Is ACCQUIREnet a Clinical Quality Program or Research?
While there are many similarities to a clinical quality program such as ACS NSQIP™ or Solutions for Patient Safety™, ACCQUIREnet is technically a research collaborative. ACCQUIREnet is registered with ClinicalTrials.gov (NCT02702869) as an observational study of short- and long-term outcomes related to multidisciplinary treatment of cleft lip/palate and craniofacial conditions. As such, team participation in ACCQUIREnet requires IRB approval and signed consent from the patient/parent. Teams that join the ACCQUIREnet collaborative will be added to the ClinicalTrials.gov listing during our annual update of the clinical trial registration.
What Data Are Collected by ACCQUIREnet?
Participating sites in ACCQUIREnet collect outcomes data and related variables that are prescribed by the ICHOM Standard Set for the Comprehensive Appraisal of Cleft Care (cf. journal article, web site). Extra outcome measures have been added to suit the needs and research priorities of the sites in ACCQUIREnet. A full listing of the variables collected appears in the ACCQUIREnet CleftCap codebook (.PDF) and data dictionary (.CSV). This dataset includes protected health information (PHI).
|
Identifiers† |
Demographics |
Clinical Features |
|---|---|---|
|
|
|
† Identifiers such as name and date of birth are used for deduplication and record linkage. Location is used for geostatistical analyses. Photographs and speech recordings are used for blinded third-party review of aesthetic and speech outcomes.
|
Outcomes |
|
|---|---|
|
Domain |
Instrument or Variables |
|
Speech |
|
|
Hearing |
|
|
Breathing |
|
|
Eating |
|
|
Dental |
|
|
Appearance |
|
|
Psychosocial |
|
|
Process metrics |
|
When Are Data Collected?
For the purpose of benchmarking and comparing outcomes, ACCQUIREnet encourages data acquisition at ages 3yo, 5yo, 8yo, 12yo, 15yo, and at the end of treatment. Other important time points are before and after important operative procedures. The CleftCap project template makes it clear which outcomes are measured at each time point.
Teams are allowed to collect data at any time points they prefer – annually or more frequently, if desired.
Who Collects the Data?
Some outcomes are measured by clinicians; others are reported by guardians (e.g., ICS) and patients (e.g., CLEFT-Q™ and FACE-Q™). Each site has formalized a workflow designating how the measurements are recorded in REDCap®. For clinical outcome measures, some sites have clinicians doing so themselves, some sites have the team coordinator entering the data, and some sites utilize research coordinators. For patient-reported and family-reported outcome measures, some sites distribute surveys ahead of clinic using REDCap®’s built-in survey capability, whereas other teams hand out the surveys on touch-screen tablet computers (like iPad®) during the clinic visit.
How Are the Data Stored, Transferred, and Utilized?
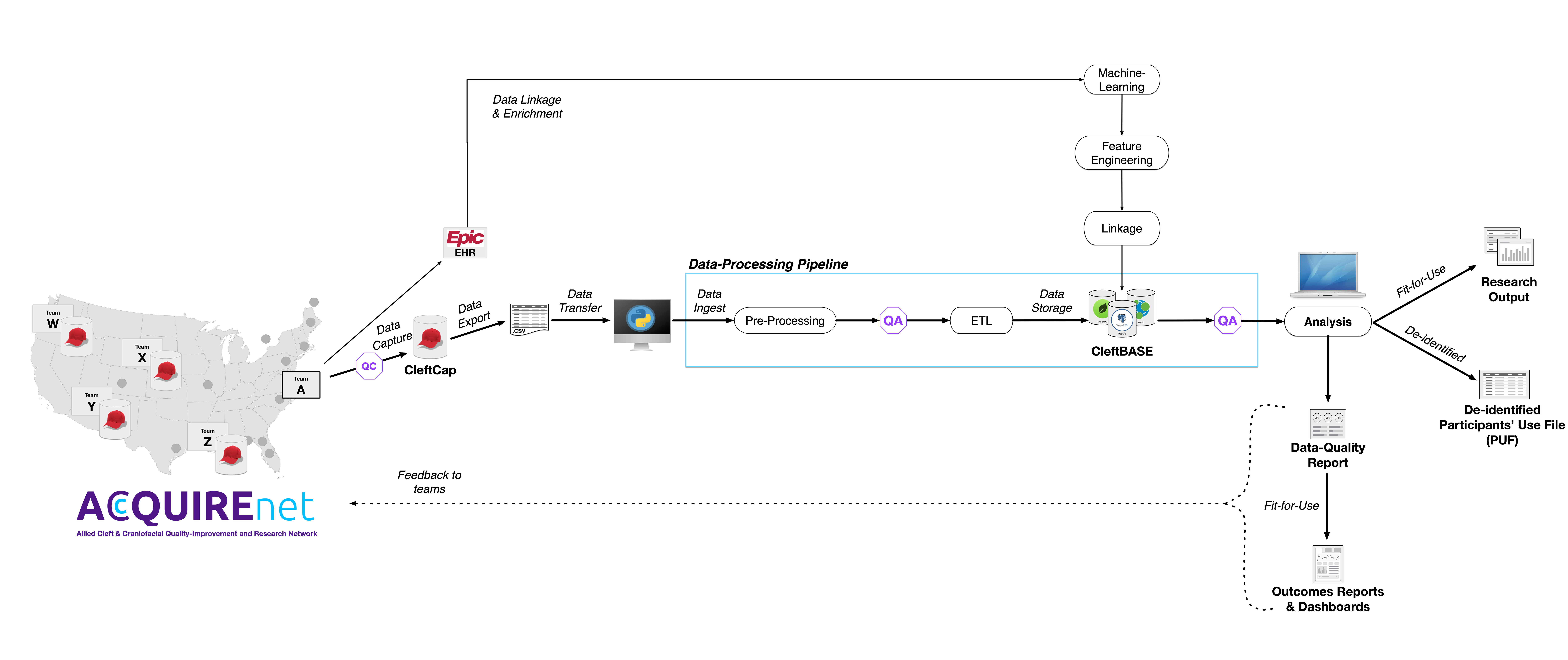
The diagram below depicts how each participating team collects and stores data, how those data are transferred to the statistical support center, and how the data are analyzed.
As part of the regular clinical workflow, each team takes care of the patient, documents the patient encounter in the electronic health record (EHR), and also (if the patient is due for outcome measurement) records the outcomes in the CleftCap database.
Local data analyses may be performed by each participating site at any time.
Periodically (usually semi-annually), the data must be aggregated from each site to permit specific analyses such as benchmarking and generation of performance reports. Duke University functions as the statistical support center for this process. Secure means of transfer is specified in the IRB protocol and data-transfer agreements with participating sites and may include secure-FTP or university-approved cloud storage system such as Box or OneDrive.
Once data are received by Duke, the data are stored in a secure enclave, which is approved for storage of PHI. The data are reformatted from the raw schema used in the CleftCap project template and transformed into a format more suitable for data analysis, using a project-specific extension to the PCORnet® Common Data Model. It is in this phase that records linkage and enrichment are possible (e.g., EHR, epidemiologic surveillance data from birth-defects monitoring programs, socioeconomic data from Census.gov, payor data from CMS and insurance carriers, etc.). Data-quality checks are performed along various steps in this pipeline. Finally, the data are stored in a secure database (CleftBASE), which is utilized for the analytical queries.
Analysis includes calculation of network benchmarks for each outcome measure. Team-specific performance reports (or quality-improvement [QI] reports) will be made available to each participating team. These reports are private and confidential — each team will only see its own performance, relative to the network benchmark, and will not be able to know the performance of any other team. For now, these reports are static reports, but a longer-term aim is to create a dynamic dashboard so that teams may explore their team’s data in different ways.
Research aims are handled similarly to QI reports: The statistical team will analyze the data in CleftBASE according to the specific aims and formal, a priori statistical analysis plan. De-identified results tables are sent to the principal investigator. (Any participant in ACCQUIREnet may be an investigator.)
A long-term aim and milestone for the network is to curate a de-identified multi-site dataset that would be made available to each participating site. This “participant use file” (PUF) is modeled after the successful practice of the American College of Surgeons (ACS) National Surgical Quality-Improvement Program (NSQIP®) and similar features in AHRQ HCUP databases. By going this extra step and publishing a de-identified multi-site dataset, participating sites would be able to perform even more analyses that might exceed the capacity of the statistical support center to handle. In this manner, our long-term vision is to make the data even more accessible for every participating center. A signed user agreement guides usage of the PUF. The PUF is only available to participating sites in ACCQUIREnet and may not be re-distributed.
Who Controls the Data?
Since ACCQUIREnet is a distributed data network, each participating team remains in full control of its own data. There is no lock-in: every team director has the ability to export and download that site’s data at any point in time. Each participating site is allowed to analyze its own data in any way it deems fit (in accordance with the IRB protocol).
Of course, for security and privacy reasons, multi-site data aggregation and analysis will only be performed by the statistical support center at Duke. Any participant in ACCQUIREnet may volunteer to be an investigator; as such, he or she would develop the specific aims and statistical analysis plan with the Duke statisticians. The statisticians would then perform the analysis and send the results to the investigator, and they would work together in the writing of the manuscript. In this manner, data security is not compromised, but every participant has the ability to work on specific areas of interest.
What About Authorship?
The core tenet of ACCQUIREnet is collaboration, not competition. Presently, we propose that any participant can propose and lead a scientific inquiry and may be first or senior author on any academic publication that results from that work. The core research team and statistical support team at Duke will also be named authors on the papers, as will any other collaborators who meet the ICMJE criteria for authorship. Certain platform papers may include all participants, listed by name wherever possible, or using the network byline “on behalf of ACCQUIREnet” where necessary according to the journal’s restrictions. Each manuscript will also include boilerplate acknowledgment statements to meet the licensing requirements of REDCap®, CLEFT-Q™ and FACE-Q™, ICHOM, and others.
What Is the Governance Structure for ACCQUIREnet?
ACCQUIREnet is a collaborative network, and every participating site may contribute to the network’s standards, areas of focus, and priorities. Every participant may pose research questions, for which he or she may serve as first or senior author. Sustaining such a network requires dedicated effort, and Duke University is serving as the network coordinating center and as the statistical support center for data analysis. Furthermore, ACCQUIREnet has a stewardship committee to assist with governance.
Which Teams Have Joined ACCQUIREnet?
As of February 2025, the following sites have joined or are in the process of joining ACCQUIREnet:
- Arnold Palmer Hospital for Children — Orlando Health (Orlando, FL)
- Boston Children’s Hospital — Harvard University (Boston, MA)
- Brenner Children’s Hospital — Wake Forest University (Winston-Salem, NC)
- Children’s Hospital Colorado (CHCO) — University of Colorado (Aurora, CO)
- Children’s Memorial Hermann Hospital — University of Texas at Houston (Houston, TX)
- Duke Children’s Hospital — Duke University (Durham, NC)
- Indiana University Health (Indianapolis, IN)
- Joe DiMaggio Children’s Hospital (Hollywood, FL)
- Johns Hopkins All Children’s Hospital — Johns Hopkins University (St. Petersburg, FL)
- Maine Medical Center (Portland, ME)
- Nicklaus Children’s Hospital (Miami, FL)
- St. Louis Children’s Hospital — Washington University (St. Louis, MO)
Additionally, ACCQUIREnet enjoys fruitful collaboration with other networks and sites that collect ICHOM and CLEFT-Q™/FACE-Q™ data, including McMaster University, Toronto SickKids, Erasmus, and Karolinska.
Does It Cost Money to Participate in ACCQUIREnet?
No. Joining ACCQUIREnet is free, and presently the project team has no plans on charging for participation. A network this size does require money to be sustained, and so far this money comes from institutional (Duke University) and grant funding.
Want to Learn More?
For additional information, please contact info@accquire.net. Our project team will reach out to you to discuss further and to answer any questions that you may have. The principal investigator, Alexander Allori, may be reached at alexander@accquire.net.
When typing the email addresses, please note that ACCQUIREnet has two Cs (abbreviating “cleft & craniofacial”).
If your team decides to join the network, you will receive an “onboarding packet” that will guide you through necessary steps. This includes the IRB protocol, starting draft for DTA, license information, a memorandum of understanding for participation, the CleftCap codebook (for implementation in REDCap®) and project template, and all other materials needed to join the network.
Acknowledgments
ACCQUIREnet is indebted to many domain experts who have generously shared their time and expertise over the years. ACCQUIREnet has been strongly inspired by Americleft, European Reference Network (ERN), EUROCRAN, ICHOM, NSQIP-Pediatric, Solutions for Patient Safety, and PCORnet®.
The principal investigator (Alexander Allori) would like to express his sincere appreciation to the following individuals:
- Jeffrey Marcus, MD, who has not only been my most influential clinical mentor, colleague, and friend, but who also scribbled on a Post-It® note: "Help me with this project. Figure out how to analyze cleft outcomes." That led to Project CHEER, CLP360°, and the rest is history.
- David Fitzsimons, PhD, who first invited me to join the ACPA Data Standards Committee in 2014, shared with me his experience building his own database, and teaching me a million things about cleft-related speech.
- John Meara, MD, DDS, MBA, for hearing about my early work in CLP360° and inviting me to be one of the co-directors of the ICHOM Standard Set working group. Without him, there would have been no Standard Set.
- Anne Klassen, PhD, and Karen Wong-Riff, MD, PhD, who kindly shared their CLEFT-Q™ and FACE-Q™ instruments, which constitute a substantial portion of the outcome metrics in our project.
- Elizabeth Heitman, PhD, who introduced me to the field of health-technology assessment. What started as a simple master's thesis has set the course for the rest of my academic career.
- Amy Abernethy, MD, PhD, who showed me how learning health systems can be utilized to study the effectiveness and quality of healthcare interventions. This led to the development of the "EQUIP model," which I have used as the underlying structure for ACCQUIREnet.
- Hayden Bosworth, PhD, who provided valuable instruction on dissemination and implementation science research methods.
- Keith Marsolo, PhD, who generously shared his insights and experiences gained from his pioneering work on PCORnet®. His contributions have been instrumental in shaping the informatical design choices that we made for ACCQUIREnet.
- Leslie Curtis, PhD, who welcomed me to the Department of Population Health Sciences and facilitated numerous introductions, expanding my network and knowledge.
- Kevin Weinfurt, PhD, who offered expert advice on the proper use of patient-reported outcome measures, ensuring that my research aligns with best practices.
- Miguel Hernán, MD, DrPH, ScM, whose brief but encouraging words gave me a much-needed boost of confidence. The task of developing and applying causal inference methods in surgery has been tough, and I deeply respect his vision, approach, and enthusiasm.
There were many more people and organizations that have helped along the way, and I thank you. Specifically, to all my students and resident trainees over the years, thank you for your hard work, enthusiasm, and company on this journey.