Duke Surgery researchers are bridging the gap between basic research and clinical application, moving gene therapy from the bench to the bedside

Assistant Professor of Surgery
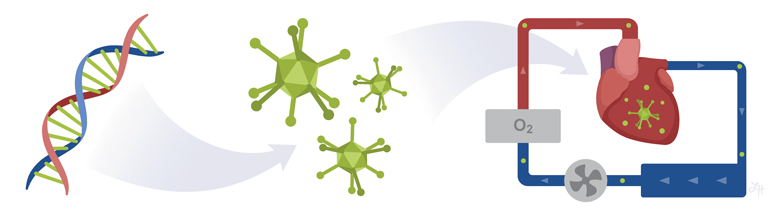
Gene therapy and gene editing platforms, such as the breakthrough technology CRISPR, have the potential to revolutionize medicine. The ability to hack into DNA and alter the genetic code to fix genetic defects opens up a new world of therapeutic applications. In the future, gene editing may help treat some of the most devastating diseases and genetic disorders facing humans.
Duke Surgery researchers are now using gene therapy and gene editing in basic research studies to evaluate potentially groundbreaking therapies for cancer, infectious disease, and transplantation.
Dawn Bowles, PhD, Assistant Professor of Surgery, Division of Surgical Sciences, in collaboration with Carmelo Milano, MD, Professor of Surgery, Division of Cardiovascular and Thoracic Surgery, and Muath Bishawi, MD, MPH, Cardiothoracic Surgery Resident, are turning to gene therapy to improve patient outcomes following heart transplantation.

Professor of Surgery
“There are still numerous negative outcomes in heart transplantation even though it’s the gold standard treatment for end-stage heart failure,” says Dr. Bowles. “Preventing organ rejection or preventing the extreme need for immunosuppression are some of the goals that we’re currently working on.”
Dr. Bowles’ team is interested in using recombinant viral vectors to deliver therapeutic genes inside the heart. These non-pathogenic viruses go door-to-door, delivering immune-altering genes to cells. Like a light switch, the genes can turn immune responses on or off. However, a successful therapy must be targeted to specific organs or tissues to avoid inadvertently triggering a detrimental immune response in other parts of the body.

Surgery Resident
Using an adenoviral vector with a luciferase indicator gene that lights up when expressed, Dr. Bowles’ team can see if the gene is localized in the heart or dispersed throughout the body. So far, this gene delivery technology has worked. The Duke Surgery team is now the first in the United States to successfully express genes in hearts using an animal model and ex vivo perfusion, a method of keeping an organ alive outside of the body.
“We’re documenting what we’re considering unprecedented global transgene expression throughout the entire heart and nowhere else in the body, which makes this approach very safe,” explains Dr. Bowles. “We feel that this is transformative because using other ways of delivering viral vectors in vivo, no one has been able to achieve what we’ve been able to achieve.”
Dr. Bowles says the next step in organ preservation is looking at cryopreserving organs to allow more time for donor matching and organ assessment. Gene therapy could be used to repair any damage done during the preservation process. She thinks CRISPR could ultimately be used to modify organs so that the antigenicity of the organ is hidden from the immune system. Cloaking an organ in an immune-altering gene could trick the body into accepting the donor organ as one of its own. This would eliminate the need for patients to take long-term immunosuppressants with toxic side effects.

With a renewed focus on using gene therapy to improve patient health, Duke Surgery recently recruited Aravind Asokan, PhD, Professor of Surgery, Division of Surgical Sciences, as Director of Gene Therapy. Dr. Asokan and his team have developed gene therapy and gene editing platforms using recombinant adeno-associated viruses to deliver therapeutic DNA to tissue and cells in preclinical animal models and patients with cancer, heart failure, muscular dystrophy, and other neuropathies.
“The broad overarching goal of the lab is to engineer viruses as tools that can enable gene editing and gene therapy platforms or modalities to move to the clinic,” says Dr. Asokan. “What that really leads to is a platform technology where you can see tailoring these delivery vehicles to target organs of interest and to match those with specific disorders and diseases.”
In partnership with Ken Poss, PhD, Director of Regeneration Next, Dr. Asokan will focus on enabling tissue regeneration to “teach the body to heal.” Gene therapy can improve wound healing for failing organs, paving the way for researchers to repair organs outside of the body by regenerating organ tissue in the lab, while surgeons reimplant the repaired organ, known as autotransplantation. In another approach, viruses would be used to deliver genetic cargo to help reprogram failing organs to heal. To overcome the organ shortage, someday researchers can grow an organ in the lab using a patient’s own cells so their immune system does not reject it.
“You have clinicians on one end in the surgery space and folks like Ken and others who are in the regeneration space, so bridging that basic science with the transformational clinical science is right where we are, in that grey zone, so that’s what makes this really exciting,” says Dr. Asokan.
In the future, rather than waiting for a transplant, patients in need of organs can simply visit their doctor’s office and have a custom-made organ ready for transplantation. While this medical frontier may seem far off, researchers in the Department of Surgery are looking to make this a reality.