Overview
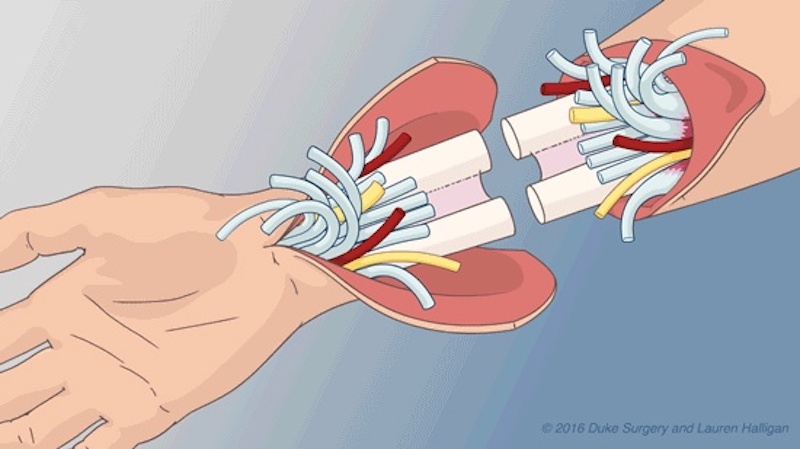
Vascularized composite allotransplantation (VCA) refers to the transplantation of multiple tissues, such as skin, muscle, tendon, nerve, and bone, as a functional unit (e.g. a hand, the abdominal wall). Several recent advances in clinical organ transplant immunosuppression and experimental VCA have now made it feasible to consider clinical VCA for functional restoration in patients with the loss of one or both hands or large tissue defects that may not be reconstructed with autologous tissue. Our research facilitates the translation of VCA from the bench to the bedside.
Key Projects Underway
- Novel immunosuppression in VCA
- Study of VCA histopathology and the scoring system for skin rejection
- Clinical hand transplantation
- Clinical abdominal wall transplantation
Visit the Duke Hand Transplant program website or the Duke Health blog to learn more about hand transplantation at Duke.
Selected Achievements
Our group has established preclinical models to understand VCA rejection in different tissues and to use that insight to minimize immunosuppression in VCA recipients who participate in clinical trials. We also organized the first public international consensus discussions conference in VCA at the Ninth Banff Conference on Allograft Pathology in Spain in 2007 resulting in the Banff VCA 2007 classification for skin allograft pathology. Additionally, we established a VCA Consortium to enable the comprehensive analysis of samples from patients in VCA clinical trials around the country.
Based on our studies of different immunosuppressive regimens in preclinical models, we have been the first to show that belatacept prevents rejection in VCA in primates and controls rejection in humans. We are currently investigating this approach in a clinical trial of hand transplant recipients (NCT02310867). This clinical trial aims to determine the safety and efficacy of hand transplantation as a treatment for patients with limb loss. This study will also test the efficacy of belatacept to prevent rejection of the transplanted hand.
Advanced Training
- Opportunity for research in VCA
Contact Us
Linda Cendales, MD
linda.cendales@duke.edu
Publications and Funded Projects
View Dr. Cendales's profile to see her publications and funded projects.