
Associate Professor in Pathology
Member of the Duke Cancer Institute
Office: LSRC B217, Durham, NC 27710
Campus Mail: DUMC LSRC B217, 308 Research Drive, Durham, NC 27710

Office: Dept of Surgery, Box 3974 DUMC, Durham, NC 27710
Overview
Adipose tissue comprises the majority of breast volume. Adipose stem cells (ASCs) are a large component of this tissue, and can differentiate into three lineages: adipocytes, osteocytes, and chondrocytes. ASCs secrete numerous cytokines that likely influence mammary gland biology, thus contributing to breast cancer initiation and/or progression.
Dr. Scott T. Hollenbeck is a plastic surgeon who performs preventive mastectomies on women at high risk for developing breast cancer. His laboratory has established a one-of-a-kind breast adipose tissue repository from these high-risk women, and they have isolated ASCs from these high-risk women to study their biology.
Dr. Robin E. Bachelder is a cancer cell biologist whose laboratory studies pathways that drive breast cancer progression and chemotherapy resistance. Based on their shared interests in reducing mortalities attributable to breast cancer, Drs. Bachelder and Hollenbeck (co-PIs) have established a collaborative program studying ASC contributions to breast cancer progression. Their goal is to identify therapies for targeting ASC signaling pathways that contribute to breast cancer initiation/progression.
- Adipose stem cell contributions to chemotherapy resistance and breast cancer recurrence
- Impact of BRCA mutations on ASC biology: Contributions to breast cancer risk
- Obesity effects on ASC cell biology: Contributions to breast cancer risk/progression
Selected Achievements
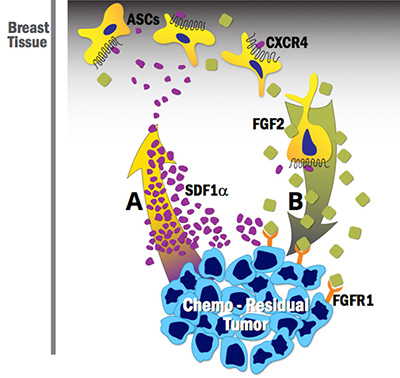
Drs. Hollenbeck and Bachelder recently co-mentored a medical student studying adipose stem cell (ASC) contributions to breast cancer recurrence post–neoadjuvant chemotherapy treatment. Through this work, they established that ASCs: 1) are recruited to chemo-residual breast cancer cells in a manner dependent on the Stromal-derived Factor 1 alpha (SDF1a)/CXCR4 chemokine axis, and 2) secrete fibroblast growth factor 2 (FGF2), which, in a paracrine manner, drives proliferation of fibroblast growth factor receptor-expressing chemo-residual breast cancer cells (See Figure 1). These findings suggest that CXCR4 or FGF receptor inhibition may prevent tumor recurrence in breast cancer patients receiving neoadjuvant chemotherapy.
- Lyes MA, Payne S, Ferrell P, Pizzo SV, Hollenbeck ST, and RE Bachelder. Adipose Stem Cell Crosstalk with Chemo-residual Breast Cancer Cells: Implications for Tumor Recurrence. In revision. Breast Cancer Research and Treatment.
Although BRCA1 mutation is known to confer genomic instability, the detailed mechanisms by which these mutations influence breast biology to drive tumor growth remain unclear. Most studies to date have focused on BRCA1 mutation effects on epithelial cell biology. However, the possibility that these mutations alter the behavior of cells in the tumor microenvironment in a manner that contributes to breast cancer risk has not been addressed.
DNA damage results in cellular senescence. Our laboratory has shown that BRCA1 knockdown adipose stem cells (ASCs) express markers of DNA damage, are senescent, and secrete cytokines [e.g. Interleukin 6 (IL6)] associated with senescence. This work has resulted in our attainment of a Duke Cancer Institute BRCA pilot award, allowing us to test the hypotheses that: 1) BRCA1 mutant ASCs can transform breast epithelial cells by supporting paracrine IL6 signaling, and 2) an FDA-approved rheumatoid arthritis drug (IL6 receptor-neutralizing antibody; Tocilizumab, Roche) blocks this paracrine signaling and prevents cellular transformation. Results from these studies have the potential ultimately to result in clinical trials testing if tocilizumab treatment of BRCA1 mutation carriers reduces breast cancer risk.
- Zhao R, Kaakaki R, Liu X, Li F, Liu L, Lee A, Bachelder R, Li, C, and ST Hollenbeck. CRISPR/Cas9-mediated BRCA1 Knockdown Adipose Stem Cells Promote Breast Cancer Progression. In Press. Plastic and Reconstructive Surgery.
Lab Members
Faculty
Trainees (Past and Current)
- Xiaoshuang Guo
- Matthew Lyes
- Jonah Orr
- Ruya Zhao
Research Associates
- Valery Nelson
Advanced Training
Opportunities are available for students and residents to work on adipose/ breast cancer-related research projects in the laboratory.
Contact Us
- Robin Bachelder, PhD, robin.bachelder@duke.edu
- Scott Hollenbeck, MD, scott.hollenbeck@duke.edu