Overview
I am a cellular immunologist with 22 years of experience in immunology and immunotherapy. My PhD training was in Dr. Barry Rouse’s lab at the University of Tennessee, Knoxville and my post-doctoral training was in Dr. Eli Gilboa’s lab at Duke University Medical Center.
The research in our laboratory focuses on the designing and testing of novel vaccines against cancer and viral infections using murine and human assay systems. In a pioneering study, our group demonstrated that dendritic cells, pulsed with unfractionated total RNA isolated from tumor cells, stimulates tumor immunity both in murine tumor models and in vitro human assays. A large number of our pre-clinical strategies have been translated into Phase I clinical trials in cancer patients. The focus and challenge of our laboratory, both at the preclinical and clinical level, is to augment the clinical benefit associated with immunotherapy. Our long-term goals are as follows: (1) evaluate the combined effects of individual strategies, (2) extend the clinical exploration to multiple cancers, and (3) combine immunotherapy and immune modulation with targeted cytotoxic therapy (radiotherapy, chemotherapy, immunotoxin therapy, and oncolytic poliovirus therapy).
Selected Achievements
In the News
- DoD Breast Cancer Program Highlight’s Nair Lab Research
- CBS 60 Minutes feature on the oncolytic poliovirus work (Part 1 and Part 2)
Accomplishments
- Researchers in the lab invented the RNA transfected dendritic cell-based vaccination approach. We have been instrumental in establishing the first GMP-production facility for dendritic cell-based cancer vaccines for Phase I clinical trials at Duke.
- Manuscript in Science Translational Medicine, Sep 20 2017, describing how poliovirus induces antitumor immunity
Research projects
1. Dendritic cells transfected with RNA as cancer vaccines
Research Question: Can we develop a novel vaccine strategy that is translatable and broadly applicable to all cancer patients, including patients with low tumor burden?
Summary: We tested the hypotheses that: 1] Immunization with a broad repertoire of tumor antigens isolated form cancer cells is superior to using defined tumor antigens. 2] Loading antigen on DCs in the form of tumor mRNA is highly effective and provides unique advantages over other forms of tumor antigen, specifically, the ability to amplify the antigenic content of a small number of tumor cells. In a pioneering study, our group demonstrated that dendritic cells loaded with unfractionated total RNA isolated from tumor cells stimulates tumor immunity both in murine tumor models and in vitro human assays. This led to the patent on the use of RNA transfected dendritic cells as vaccines (awarded in 1998). In 1999, Argos Therapeutics was established in Durham, NC to commercialize this approach.
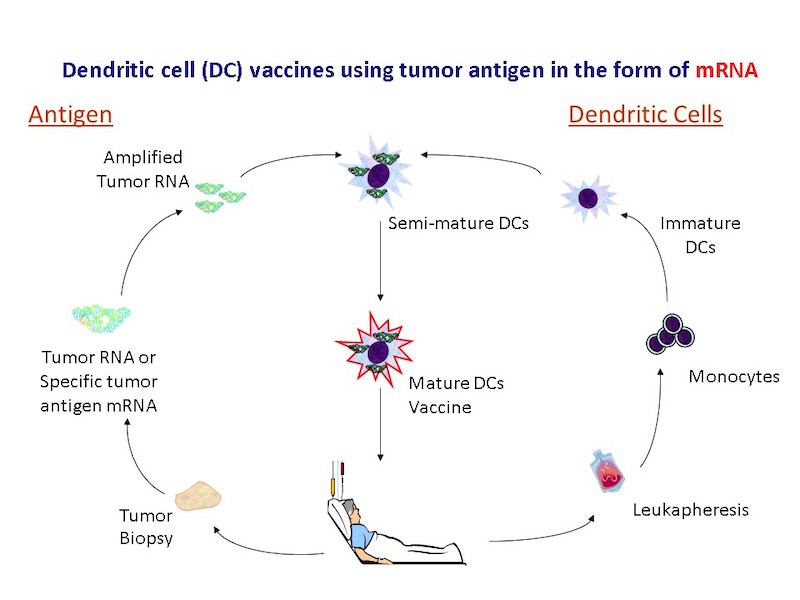
2. Modulation of immune responses to enhance tumor-specific immunity
Research Question: How can the therapeutic benefit for patients treated with RNA-transfected dendritic cells be improved?
Summary: Modulating immune responses by blocking inhibitory immune receptors and activating stimulatory immune receptors on T cells enhances tumor-specific immunity. Systemic administration of antibodies (Abs) targeting inhibitory immune receptors enhanced the stimulation of immune responses in mice. However, clinical use of such Abs is limited by toxicity. We have developed an approach for local modulation of immune responses by delivering RNA encoding immune modulating proteins (Abs or receptor-binding ligands) regionally at the site of T cell activation.
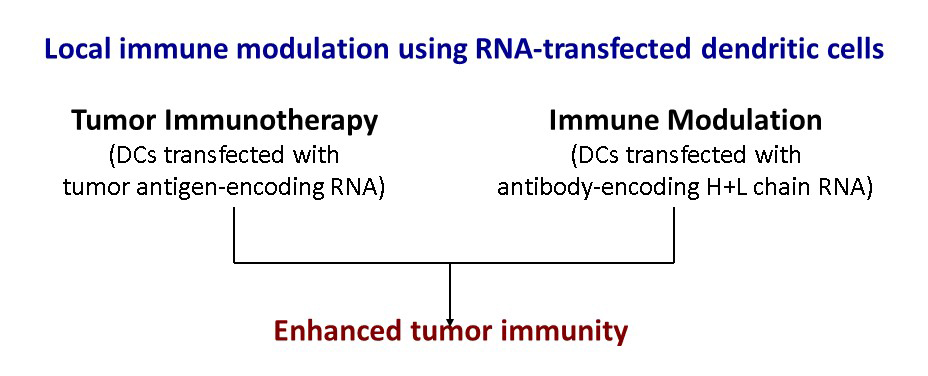
3. Novel vaccine strategies
Research Question: How do we develop an effective vaccine strategy that is NOT time-, labor-, or resource-intensive?
Summary: A drawback of dendritic cell-based vaccination is that the process of harvesting, culturing, and loading dendritic cells with antigens is time-, labor-, and resource-intensive. We have demonstrated an innovative method of vaccination as a proof-of-concept in a study by loading mRNA encoding a tumor antigen onto whole blood cells. This whole blood cell mRNA vaccine is as potent as the dendritic cell RNA vaccine in a mouse tumor model of melanoma.
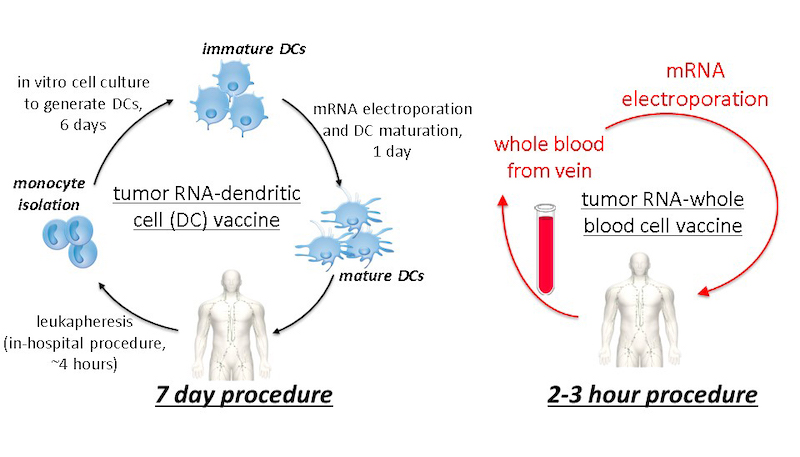
4. Oncolytic poliovirus immunotherapy
Research Question: Does regional cytoxicity with oncolytic poliovirus stimulate innate immune events that promote an in situ vaccine effect?
Summary: Matthias Gromeier, PhD, has pioneered an oncolytic poliovirus (OncPV) therapy, PVSRIPO, which selectively targets and eliminates malignant cells. A first-in-human Phase-1 study with PVSRIPO at Duke University has shown remarkable promise in patients with recurrent glioblastoma multiforme (GBM), a uniformly lethal disease. In collaboration with the Gromeier lab we are testing the following hypothesis: 1] regional OncPV therapy initiates immune events associated with oncolysis and inflammation; 2] these immune events could potentially lead to an in situ vaccine effect and a systemic immune response. We will test this hypothesis in immunocompetent mouse models.
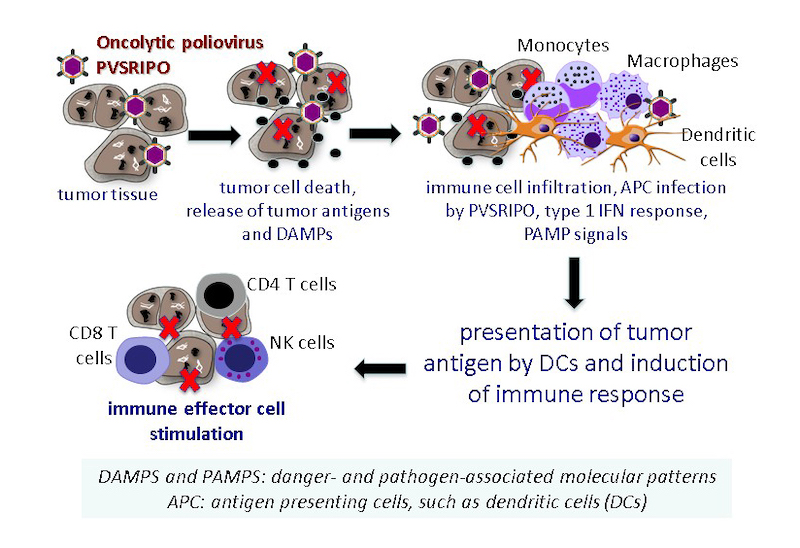
Current research in the Immunology, Inflammation, and Immunotherapy Laboratory examines:
- Dendritic cell vaccines using tumor-antigen encoding RNA (mRNA, total tumor RNA, amplified tumor mRNA)
- Local immune receptor modulation using mRNA that encodes for antibodies, receptor-ligands, cytokines, chemokines, and Toll-like receptors (current target list: CTLA4, GITR, PD1, TIM3, LAG3, OX40, and 41BB)
- Combination therapies for cancer: cytotoxic therapy (radiation, chemo, and oncolytic poliovirus therapy) with dendritic cell-based vaccines and immune checkpoint blockade
- Adoptive T cell therapy using tumor RNA-transfected dendritic cells to expand tumor-specific T cells ex vivo
- Adoptive T cell therapy using PSMA CAR (chimeric antigen receptor) RNA-transfected T cells
- Direct injection of tumor antigen encoding RNA (targeting antigens to dendritic cells in vivo using nanoparticles and aptamers)
Publications and Funded Projects
View Dr. Nair's profile to see her publications and funded projects.
Lab Members
Faculty Members
Post-Doctoral Fellows
- Jiyoung (Sarah) Ahn, PhD
- Michael C. Brown, PhD
- Austin Eckhoff, MD
- Ibtehaj (Janoo) Naqvi, MD, PhD
- Adam Swartz, PhD
Undergraduate Student
- Lauren Y. Sheu
Duke University '22
Lab Personnel
- David Boczkowski, MS
Associate in Research - Victoria Frazier
Research Analyst - David Snyder
Research Analyst
Publications
Below are selected peer-reviewed publications, categorized by research topic. See a full list of Dr. Nair's publications.
Dendritic Cells Transfected with RNA as Cancer Vaccines
Nair SK, Boczkowski D, Morse M, Cumming RI, Lyerly HK, Gilboa E. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nature Biotechnology 1998;16(4):364-9. PMID: 9555728.
Nair SK, Heiser A, Boczkowski D, Majumdar A, Naoe M, Lebkowski JS, Vieweg J, Gilboa E. Induction of cytotoxic T cells and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nature Medicine 2000;6(9):1011-7. PMID: 10973321.
Boczkowski D, Nair SK, Nam JH, Lyerly HK, Gilboa E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Research 2000;60(4):1028-34. PMID: 10706120.
Nair S, Boczkowski D, Moeller B, Dewhirst M, Vieweg J, Gilboa E. Synergy between tumor immunotherapy and antiangiogenic therapy. Blood 2003;102(3):964-71. PMID: 12689940.
Nair SK, De Leon G, Boczkowski D, Schmittling R, Xie W, Staats J, Liu R, Johnson LA, Weinhold K, Archer GE, Sampson JH, Mitchell DA. Recognition and killing of autologous, primary glioblastoma tumor cells by human cytomegalovirus pp65-specific cytotoxic T cells. Clinical Cancer Research 2014. 2014 May 15;20(10):2684-94. PMID: 24658154
Modulation of Immune Responses to Enhance Tumor-Specific Immunity
Nair S, Boczkowski D, Fassnacht M, Pisetsky D, Gilboa E. Vaccination against the forkhead transcription factor Foxp3 enhances tumor immunity. Cancer Research 2007;67(1):371-80. PMID: 17210720.
Boczkowski D, Lee J, Pruitt S, Nair S. Dendritic cells engineered to secrete anti-GITR antibodies are effective adjuvants to dendritic cell-based immunotherapy. Cancer Gene Therapy 2009;16(12):900-11. PMID: 19498460.
Pruitt SK, Boczkowski D, de Rosa N, Haley NR, Morse MA, Tyler DS, Dannull J, Nair S. Enhancement of anti-tumor immunity through local modulation of CTLA-4 and GITR by dendritic cells. Eur J Immunol 2011;41(12):3553-63. Epub 2011/10/27. doi: 10.1002/eji.201141383. PMID: 22028176.
Pratico ED, Sullenger BA, Nair SK. Identification and characterization of an agonistic aptamer against the T cell costimulatory receptor, OX40. Nucleic Acid Therapeutics 2013 Feb;23(1):35-43. PMID: 23113766
Novel Vaccine Strategies
Phua KK, Leong KW, Nair SK. Transfection efficiency and transgene expression kinetics of mRNA delivered in naked and nanoparticle format. Journal of Controlled Release 2013 Mar 28;166(3):227-33. doi: 10.1016/j.jconrel.2012.12.029. Epub 2013 Jan 7. PMID: 23306021
Phua KK, Boczkowski D, Dannull J, Pruitt S, Leong KW, Nair SK. Whole blood cells loaded with messenger RNA as an anti-tumor vaccine. Advanced Healthcare Materials 2014 Jun;3(6):837-42. doi: 10.1002/adhm.201300512. PMID: 24339387
Phua KK, Staats HF, Leong KW, Nair SK. Intranasal mRNA nanoparticle vaccination induces prophylactic and therapeutic anti-tumor immunity. Scientific Reports 2014;4:5128. PMID: 24894817.
Laboratory-to-Clinic Translation Studies (Team Science)
Nair SK, Morse M, Boczkowski D, Cumming RI, Vasovic L, Gilboa E, Lyerly HK. Induction of tumor-specific cytotoxic T lymphocytes in cancer patients by autologous tumor RNA-transfected dendritic cells. Annals of Surgery 2002;235(4):540-9. PMID: 11923611.
Morse MA, Nair SK, Mosca PJ, Hobeika AC, Clay TM, Deng Y, Boczkowski D, Proia A, Neidzwiecki D, Clavien PA, Hurwitz HI, Schlom J, Gilboa E, Lyerly HK. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Investigation 2003;21(3):341-9. PMID: 12901279.
Dannull J, Haley NR, Archer G, Nair S, Boczkowski D, Harper M, De Rosa N, Pickett N, Burchette J, Selim MA, Mitchell D, Sampson J, Tyler DS, Pruitt SK. Altering the proteasome of dendritic cells used for vaccination enhances anti-tumor immunity in melanoma patients. Journal of Clinical Investigation 2013 Jul;123(7):3135-45. PMID: 23934126
Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, Congdon KL, Reap EA, Archer GE, Desjardins A, Friedman AH, Friedman HS, Herndon JE 2nd, Coan A, McLendon RE, Reardon DA, Vredenburgh JJ, Bigner DD, Sampson JH. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 2015 Mar 19;519(7543):366-9. PMID: 25762141
