Overview
Dr. Lidsky is the Director of HPB Research at Duke University. He leads the Surgical Oncology Research Group, which was initiated by Dr. Blazer. This group, consisting of surgical oncology faculty, surgery trainees, and medical students, meets monthly to review ongoing research projects that utilize local and national datasets to study cancers of the liver, biliary tree, and pancreas, gastric cancer, soft tissue sarcoma, peritoneal malignancies, and melanoma, in additional to pre-malignant conditions such as intrapapillary mucinous neoplasms. This platform is also utilized for educational activities, including invited speakers on topics such as statistical methods, clinical trial methodology, and data warehouse search engines.
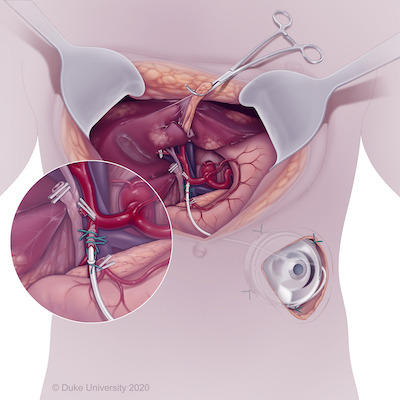
Upon joining the faculty in 2018, Dr. Lidsky implemented and continues to lead the Hepatic Artery Infusion (HAI) team at Duke, a rapidly growing program to deliver high-dose chemotherapy directly to the liver for patients with advanced primary and secondary liver cancers (https://www.dukehealth.org/blog/chemotherapy-pump-helps-people-metastati...). HAI entails the surgical implantation of a subcutaneous pump with a catheter that is inserted into the hepatic arterial system, allowing for the delivery of high-dose chemotherapy directly to the liver, typically given concurrently with systemic therapy. The primary agent delivered via HAI is floxuridine (FUDR), which has the ideal properties of a short half-life (<10 minutes) and near-complete hepatic clearance, such that drug concentrations in the liver reach up to 400 times that which could be achieved with intravenous delivery, but without systemic exposure/toxicity. HAI is most often considered for patients with unresectable colorectal liver metastases, with the goal of controlling disease or converting patients to resectable status, as adjuvant therapy after resection of colorectal liver metastases to delay or prevent hepatic recurrence, and in the management of locally unresectable intrahepatic cholangiocarcinoma. Parallel to this effort, Dr. Lidsky co-founded and co-leads the international HAI Consortium Research Network (HCRN), a large collaborative of over 50 centers working together to improve HAI outcomes and develop and implement modern-day multicenter prospective HAI trials.
Dr. Lidsky is also a surgeon-scientist, where his basic science and translational research is conducted in Dr. Kris Wood’s laboratory within the Department of Pharmacology and Cancer Biology. His work focuses on determining mechanisms of tumorigenesis and therapeutic resistance in intrahepatic cholangiocarcinoma, so as to identify novel therapeutic strategies for this deadly malignancy. Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer in the United States, with a 128% increase in incidence in the last four decades. The disease is highly aggressive and lethal, with only 20% of patients qualifying for curative intent resection. Currently, very few treatments for ICC exist, particularly with regards to systemic therapy. While adjuvant capecitabine yields modest improvements in survival, most ICC patients present with advanced disease for which prognosis is exceedingly poor, with median survival under 1 year.
Complicating advancement is the lack of relevant and reliable pre-clinical models of ICC. To circumvent this shortcoming in available models, Dr. Lidsky is using patient samples to derive a gold-standard library of novel ICC models, including cell lines, organoids, and xenografts. He then applies functional genomics and precision pharmacology techniques, including unbiased CRISPR/Cas9 screening, RNAi, and high-throughput small molecule screens, to identify tumor-specific dependencies that may be exploited as novel therapeutic strategies.
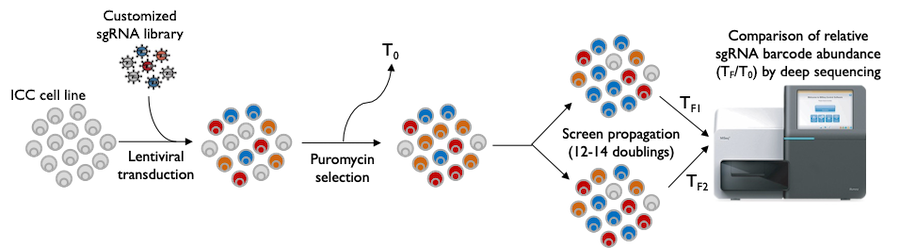
Further, worldwide efforts focused on tumor sequencing and mutation-based therapies have shown only modest improvements in survival. Within the context of genomic alterations, FGFR2 fusions are detected in approximately 15% of ICC patients and can be treated with novel agents, although these drugs are neither universally effective nor capable of long-lasting control of the cancer. To learn more about this subtype of ICC, Dr. Lidsky has generated unique pre-clinical models from an ICC patient harboring an FGFR2 fusion, which he leverages to learn about FGFR2 biology, identify new treatment strategies that together with available agents can improve effectiveness, as well as detect patterns of drug resistance that ultimately develop so as to delay or overcome this inevitable process that ends in disease progression.
Dr. Lidsky has received numerous grants to support his research, including the Duke Physician-Scientist Strong Start Award, American College of Surgeons National Surgeon Scientist Program, The Cholangiocarcinoma Foundation Research Fellowship, and Duke CTSA KL2 Award.
Publications and Funded Projects
View Dr. Lidsky's profile to see his publications and funded projects.