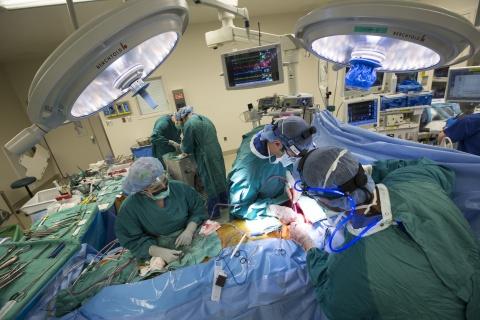
Photo: Cardiothoracic surgeons Dr. Jacob Klapper and Dr. Nnamdi Nwaejike perform a double lung transplant. Shawn Rocco/Duke Health
Each day, tens of thousands of patients on waiting lists across the United States await a simple phone call: one that says a match has been found and an organ is available for transplant. Despite a growing demand for donors, organ shortages continue to hinder many patients’ chances in receiving their potentially life-saving call.
The organ shortage has impacted several transplant teams at Duke. Carmelo Milano, MD, Professor, Division of Cardiovascular and Thoracic Surgery, says part of the reason for the shortage is the method used to preserve organs while in transit from donor to recipient.
“Duke has performed over 1,000 heart transplants using cold static storage,” says Dr. Milano, Heart Transplant Surgical Director. “With this method, the heart is removed from the donor and cooled with a solution before it is transported, but lack of oxygen to the organ can cause graft failure.”
Cold storage has been a staple of the transplant procedure for over 50 years. While this method is serviceable, it is far from ideal. Vital organs are sensitive to cold ischemia while stored on ice, as irreparable damage from a lack of oxygenated blood flow rapidly occurs. When a heart stops beating, or lungs stop breathing, the organ slowly dies.
The cold slows down the deterioration process but not entirely, and this race against the clock severely limits organ availability. The heart is particularly sensitive to damage; Duke’s range of potential donors for heart transplants is limited to those east of the Mississippi, according to Dr. Milano.
The lung transplant team faces similar obstacles, says Matthew Hartwig, MD, Associate Professor, Division of Cardiovascular and Thoracic Surgery. Though the team has successfully transplanted lungs held in cold storage for longer than the conservative 4-hour window, Dr. Hartwig says doing so can create more complications. With such a small window of time, organ matches found a considerable distance away often go unused.
The Ice Age May Be Over
The most logical answer to the cold storage problem is also the most challenging: keep a transplanted organ in a near-physiologic state while outside of the body, perfused with blood, and limit the amount of time the organ is kept on ice.
Though the ability to keep an organ alive outside of the body may sound like something out of science fiction, a perfusion system makes this a reality. The device keeps an organ as fully functional as possible after surgical removal, during a process known as ex vivo perfusion. Rather than on ice, the device stores the organ at a normothermic temperature, causing less injury. Nutrient-rich blood taken from the donor filters through the organ, and the system allows close monitoring while the donated organ remains in a living state: beating, breathing, or functional.
Several Duke teams are at the forefront of national clinical studies to examine the efficacy of these devices in transplant procedures. The cardiac transplant team has partnered with TransMedics®, whose portable Organ Care System™ (OCS™) allows the heart to be perfused while in transit from donor to recipient. Dubbed the “heart in a box,” the device is sent with the procurement team to retrieve the heart, which is transported back to the transplant center. The first successful surgery at Duke using an organ transported via the OCS™ was performed in July 2016. The surgery would not have been possible without the new system due to the distance the organ had to travel, says Dr. Milano. Since then, surgeons have performed 8 more successful transplants using the OCS™.
The lung transplant team has also had success using a device developed by XVIVO Perfusion, completing 25 successful transplants and enrolling more patients than any other center in the United States for the trial. Lungs are fragile organs at high risk for infection due to their role as a main filter of our outside environment. This fragility comes at a cost: the Organ Procurement and Transplantation Network reports only 1 in 4 donated lungs is viable for transplant. Duke’s trial with the XVIVO system focused specifically on lungs that initially would not have been accepted for transplant. Using a perfusion device allows the lungs to breathe without straining, making the organ stronger and healthier outside of the body.
The liver transplant team formed a third partnership with OrganOx. The manufacturer’s metra® device allows the donor’s liver to be preserved for up to 24 hours prior to transplant. This clinical trial began in February of 2017.
“There are several benefits to using the device,” explains Andrew Barbas, MD, Assistant Professor, Division of Abdominal Transplant Surgery. “We can access the metabolic activity of the liver and restore energy levels in liver cells. We can also assess which organs will function better after transplant.”
This evaluation period prior to the transplant procedure can be critical for a successful outcome, and perfusion devices offer the surgeon more breathing room to examine the organ fully before surgery begins.
Expanding the Donor Pool
When asked to share the overall goal of using perfusion systems in transplantation, all surgeons had the same answer: to increase the number of organs available. Longer preservation times allow organs to travel greater distances, offering a larger geographic area to search for matches.
More important than geography is the ability to use “extended criteria organs,” says Jacob Schroder, MD, Assistant Professor, Division of Cardiovascular and Thoracic Surgery.
“Extended criteria hearts have some feature that makes them imperfect—ventricular hypertrophy, minor coronary disease, advanced age of the donor, certain causes of death, or a long estimated ischemic time,” says Dr. Schroder. “The success rate for these hearts has traditionally been very low, but the OCS™ device allows us to take the ischemic time out of the question.”
Whether heart, lung, or liver, when the threat of damage from cold storage is minimized, more organs become viable options for patients in need. To put it simply, Dr. Schroder explains that utilizing the OCS™ is the equivalent of transplanting an organ from a donor found in Raleigh, rather than farther away. Less risk, and more positive outcomes.
The Future of Ex Vivo Perfusion
While organ preservation was the main goal of cold storage, perfusion raises an interesting question about the ability to rehabilitate organs while outside of the body. Is it possible to transplant an organ in a better condition than when it was procured?
Dawn Bowles, PhD, Assistant Professor, Division of Surgical Sciences, believes this may be a possibility. She has conducted biological therapy studies using porcine hearts perfused in the TransMedics® OCS™.
“These devices answer questions about whether or not a heart can be rehabilitated while it is stored,” says Dr. Bowles. “It is possible that we could fix some things that need fixing in a heart before it is transplanted.”
While this type of therapy is still on the horizon, it highlights the potential of the new technology. Through a grant from the American Society of Transplant Surgeons, Dr. Barbas is using animal models to test the possibility of repairing damaged livers through perfusion. Repairing organs ex vivo may be another solution to the organ shortage problem.
This potential application may already be a reality for lung transplants. Duke’s next trial with United Therapeutics will test the use of what Dr. Hartwig calls an “organ hospital”—a centralized location where organs are rehabilitated before transplantation.
“This technology is still in its infancy, but I can see our program being able to use the devices to not only stabilize lungs while outside of the body, but to intervene and improve them,” says Dr. Hartwig.
In the end, healthier organs in greater number available for transplant means more patients receiving the life-saving operations they need. For many patients awaiting a transplant, the wait for their phone call may be a short one.